PACE trial: Difference between revisions
(remove cleanup notice, web links updated. All these web links should really be converted to CS1 citations.) |
Notjusttired (talk | contribs) m (Text replacement - " first = " to " first = ") |
||
| (253 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
[[File:Pace trial.png|right|300px]]'''The PACE Trial''' (short for "'''P'''acing, graded '''A'''ctivity, and '''C'''ognitive behaviour therapy; a randomised '''E'''valuation")<ref name=" | {{Cleanup | reason = Too long. Please consider splitting content into other articles, or condensing it | date = 2022}} | ||
[[File:Pace trial.png | right | 300px]]'''The PACE Trial''' study (short for "'''P'''acing, graded '''A'''ctivity, and '''C'''ognitive behaviour therapy; a randomised '''E'''valuation") was a large and controversial trial of treatments for people with [[chronic fatigue syndrome | Chronic Fatigue Syndrome]] (CFS), also known as [[myalgic encephalomyelitis | Myalgic Encephalomyelitis]] (ME).<ref name="PACE-protocol">{{Cite journal | last = White | first = Peter D. | authorlink = Peter White | last2 = Sharpe | first2 = Michael C. | author-link2 = Michael Sharpe | last3 = Chalder | first3 = Trudie | author-link3 = Trudie Chalder | last4 = DeCesare | first4 = Julia C. | last5 = Walwyn | first5 = Rebecca | last6 = PACE trial group | author-link6 = PACE Trial Management Group | date = 2007-03-08 | title = Protocol for the PACE trial: a randomised controlled trial of adaptive pacing, cognitive behaviour therapy, and graded exercise, as supplements to standardised specialist medical care versus standardised specialist medical care alone for patients with the chronic fatigue syndrome/myalgic encephalomyelitis or encephalopathy | url = https://www.ncbi.nlm.nih.gov/pubmed/17397525 | journal = BMC neurology | volume = 7 | pages = 6 | doi = 10.1186/1471-2377-7-6 | issn = 1471-2377 | pmc = 2147058 | pmid = 17397525 | issue = | via = | quote = | author-link4 = Julia DeCesare | author-link5 = Rebecca Walwyn}}</ref> | |||
The | The PACE trial compared standardised specialist medical care (SMC) alone to SMC plus [[adaptive pacing therapy | Adaptive Pacing Therapy]] (APT), [[cognitive behavioral therapy | Cognitive Behavioral Therapy]] (CBT), or [[graded exercise therapy | Graded Exercise Therapy]] (GET). The researchers that conducted the trial expected that the CBT and GET groups would see the greatest improvement,<ref name="PACE-protocol" /> and this finding was reported in subsequent articles published by them.<ref name="pace2011a" /><ref name="pace2013a" /><ref name="pace2015FU" /> This claim has been controversial, however, due to patient groups finding that [[exercise]] made patients deteriorate and CBT lacked benefit, and concerns regarding the methodology and refusal to release the full data.<ref name="MEACTIONpacepetition">{{citation | last1 = #MEAction | authorlink1 = #MEAction | title = Petition: Misleading Claims Should Be Retracted | website = [[The MEAction Network]] | date = Oct 2015 | url = http://my.meaction.net/petitions/pace-trial-needs-review-now | archive-url = http://web.archive.org/web/20190318172536/http://my.meaction.net/petitions/pace-trial-needs-review-now | archive-date = March 19, 2019 }}</ref><ref name="MEASSUK20150529survey" /><ref name="openletrLANCET1" /> Some patients campaigned for the full release of the PACE study's results, and after a five year battle, the PACE trial data was released for others to analyse.<ref name="iNews20160819PG" /> The full PACE data led to over 40 scientists writing an open letter to the ''Lancet'' to raise serious concerns about the study, and asking for a new, independent analysis of results.<ref name="openletrLANCET1" /> | ||
The PACE trial | The PACE trial has been highly influential in clinical guidelines in the [[United Kingdom | UK]], and other countries, in both government funded health care and private medical insurance, and had a significant influence on the [[Cochrane]] reviews for ME/CFS treatments.<ref name="NICEguideCG53" /><ref name="SwissRe2011" /><ref name="SwissRe2011b" /> By the time the main PACE trial results were published, the UK had included CBT and GET in clinical guidance for four years - the PACE trial was expected to confirm that they were effective.<ref name="NICEguideCG53" /> | ||
==Background== | ===Background=== | ||
The PACE trial<ref name="pace2011a" /> was funded by the UK [[Medical Research Council]], [[Department of Health and Social Care (UK)]] for England, [[Scottish Chief Scientist Office]], and - apparently uniquely for a clinical trial - the [[Department for Work and Pensions]] - the government department for sickness, disability and pension benefits. The PACE trial cost £5 million<ref name="RAE2008cost" /> and is the most expensive piece of research into [[ME/CFS]] ever conducted. | The PACE trial<ref name="pace2011a" /> was funded by the UK [[Medical Research Council]], [[Department of Health and Social Care (UK)]] for England, [[Scottish Chief Scientist Office]], and - apparently uniquely for a clinical trial - the [[Department for Work and Pensions]] - the government department for sickness, disability and pension benefits. The PACE trial cost £5 million<ref name="RAE2008cost" /> and is the most expensive piece of research into [[ME/CFS]] ever conducted. | ||
Recruitment of patients began in March 2005 and data collection was completed in January 2010.<ref name="pace2011a" /> The study protocol was published in BMC Neurology in 2007.<ref name="pace2007protocol" /> The main study outcomes were published in [[The Lancet]] in 2011<ref name="pace2011a" /> and the experimenters | Recruitment of patients began in March 2005 and data collection was completed in January 2010.<ref name="pace2011a" /> The study protocol was published in BMC Neurology in 2007.<ref name="pace2007protocol" /> The main study outcomes were published in [[The Lancet]] in 2011<ref name="pace2011a" /> and the experimenters continued to [[#List_of_PACE_trial_publications | publish papers on the PACE trial]] for a number of years. | ||
The principal investigators were Professors [[Peter White]], [[Trudie Chalder]], and [[Michael Sharpe]]. Although not an author, Professor [[Simon Wessely]] provided feedback on their report.<ref name="pace2011a" /> He stated in November 2015 that "there are also more trials in the pipeline".<ref name="paceWesselyTitanic" /> | The principal investigators were Professors [[Peter White]], [[Trudie Chalder]], and [[Michael Sharpe]]. Although not an author, Professor [[Simon Wessely]] provided feedback on their report.<ref name="pace2011a" /> He stated in November 2015 that "there are also more trials in the pipeline".<ref name="paceWesselyTitanic" /> | ||
==Study design== | ===Study design=== | ||
641 patients were randomised into four groups in the study.<ref name="pace2011a" /> All received specialist medical care (SMC), which consisted of medication for symptoms such as insomnia and pain, and general advice to avoid extremes of rest and inactivity.<ref name="PACEmanualSSMC" /> One group received SMC alone. Patients in another group additionally received [[adaptive pacing therapy]] (APT), and were advised to stay within the limits of activity imposed by the disease to give their bodies the best chance of recovery.<ref name="PACEmanualPAPT" /> The other two groups were both told that they were not ill but [[Deconditioning|deconditioned]], and that if they gradually increased their activity, there was nothing to prevent their recovery.<ref name="PACEmanualPCBT" /><ref name="PACEmanualPGET" /> The [[Cognitive behavioral therapy|cognitive behavioural therapy]] (CBT) group focused on addressing their presumed [[Illness beliefs|fear of activity]] while the [[graded exercise therapy]] (GET) group focused on increasing their activity in a structured manner, with regular aerobic exercise as the eventual goal. | 641 patients were randomised into four groups in the study.<ref name="pace2011a" /> All received specialist medical care (SMC), which consisted of medication for symptoms such as insomnia and pain, and general advice to avoid extremes of rest and inactivity.<ref name="PACEmanualSSMC">{{citation | last1 = PACE Trial Management Group | authorlink1 = PACE Trial Management Group | title = PACE - Manual for Doctors - Standardised Specialist Medical Care (SSMC) | date = Dec 2, 2004 | url = https://www.qmul.ac.uk/wolfson/media/wolfson/current-projects/7.ssmc-doctor-manual.pdf}}</ref> One group received SMC alone. Patients in another group additionally received [[adaptive pacing therapy]] (APT), and were advised to stay within the limits of activity imposed by the disease to give their bodies the best chance of recovery.<ref name="PACEmanualPAPT">{{citation | last1 = PACE Trial Management Group | authorlink1 = PACE Trial Management Group | url = https://www.qmul.ac.uk/wolfson/media/wolfson/current-projects/2.apt-participant-manual.pdf | title = PACE - Manual for Participants - Adaptive Pacing Therapy (APT) for CFS/ME | date = Nov 2004}}</ref> The other two groups were both told that they were not ill but [[Deconditioning | deconditioned]], and that if they gradually increased their activity, there was nothing to prevent their recovery.<ref name="PACEmanualPCBT">{{citation | last1 = PACE Trial Management Group | authorlink1 = PACE Trial Management Group | url = https://www.qmul.ac.uk/wolfson/media/wolfson/current-projects/4.cbt-participant-manual.pdf | title = PACE - Manual for Participants - Cognitive Behaviour Therapy for CFS/ME | date = Nov 2004}}</ref><ref name="PACEmanualPGET">{{citation | last1 = PACE Trial Management Group | authorlink1 = PACE Trial Management Group | url = https://www.qmul.ac.uk/wolfson/media/wolfson/current-projects/6.get-participant-manual.pdf | title = PACE - Manual for Participants - Graded Exercise Therapy for CFS/ME | date = Nov 2004}}</ref> The [[Cognitive behavioral therapy | cognitive behavioural therapy]] (CBT) group focused on addressing their presumed [[Illness beliefs | fear of activity]] while the [[graded exercise therapy]] (GET) group focused on increasing their activity in a structured manner, with regular aerobic exercise as the eventual goal. | ||
Patients in the APT, CBT and GET groups were offered up to 14 sessions with a therapist over a six-month period, to support them in following their therapy programmes, with a top-up session at 36 weeks. They also received a lengthy manual<ref name="PACEmanualPAPT" /><ref name="PACEmanualPCBT" /><ref name="PACEmanualPGET" /> explaining their therapy. All participants were offered at least three sessions of SMC. | Patients in the APT, CBT and GET groups were offered up to 14 sessions with a therapist over a six-month period, to support them in following their therapy programmes, with a top-up session at 36 weeks. They also received a lengthy manual<ref name="PACEmanualPAPT" /><ref name="PACEmanualPCBT" /><ref name="PACEmanualPGET" /> explaining their therapy. All participants were offered at least three sessions of SMC. | ||
| Line 25: | Line 24: | ||
Patients were also followed up (using subjective ratings only) at least two years after randomisation.<ref name="pace2015FU" /> | Patients were also followed up (using subjective ratings only) at least two years after randomisation.<ref name="pace2015FU" /> | ||
As | As of May 7, 2018, the PACE manuals were not retrievable from the QMUL website, but they were retrievable as of 16 July 2022.<ref>{{cite web | url = https://www.qmul.ac.uk/wiph/centres/centre-for-psychiatry-and-mental-health/research/pace-trial/ | title = PACE Trial | publisher = Queen Mary University Of London | access-date = July 16, 2022}}</ref> | ||
All documents pertaining to the PACE trial can be found following the link ''PACE trial documents''. | All documents pertaining to the PACE trial can be found following the link ''PACE trial documents''. | ||
{{Main article |page_name = PACE trial documents}} | {{Main article | page_name = PACE trial documents}} | ||
==Findings== | ===Findings=== | ||
The trial's results showed that patients in the CBT and GET groups improved more in self-rated fatigue and physical function than the APT or SMC-only groups.<ref name="pace2011a" /> Apart from the GET group improving slightly more than the others | The trial's results showed that patients in the CBT and GET groups improved more in self-rated fatigue and physical function than the APT or SMC-only groups.<ref name="pace2011a" /> Apart from the GET group improving slightly more than the others on the six-minute walking test,<ref name="pace2011a" /> all of the study's objective measures<ref name="pace2012CE">{{cite journal | last1 = McCrone | first1 = P | authorlink1 = Paul McCrone | last2 = Sharpe | first2 = M | authorlink2 = Michael Sharpe | last3 = Chalder | first3 = T | authorlink3 = Trudie Chalder | last4 = Knapp | first4 = M | authorlink4 = Martin Knapp | last5 = Johnson | first5 = AL | authorlink5 = Anthony Johnson | last6 = Goldsmith | first6 = K | authorlink6 = Kimberley Goldsmith | title = Adaptive Pacing, Cognitive Behaviour Therapy, Graded Exercise, and Specialist Medical Care for Chronic Fatigue Syndrome: A Cost-Effectiveness Analysis | journal = PLoS One | date = Aug 1, 2012 | pmid = 22870204 | doi = 10.1371/journal.pone.0040808 | url = http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0040808}}</ref><ref name="pace2015SMA" /> and the long-term follow-up data<ref name="pace2015FU" /> (self-ratings of fatigue and physical function) showed no difference between groups. | ||
The authors reported, in a 2013 paper specifically about recovery, that 22% of patients in the CBT and GET groups had recovered following these therapies, compared to 8% in the APT group and 7% in the SMC-only group. | The authors reported, in a 2013 paper specifically about recovery, that 22% of patients in the CBT and GET groups had recovered following these therapies, compared to 8% in the APT group and 7% in the SMC-only group. | ||
==Impact== | ===Impact=== | ||
The PACE trial and other studies that use the [[Oxford criteria]] for diagnosis of ME/CFS have had major international impact on popular perceptions of the disease and | The PACE trial and other studies that use the [[Oxford criteria]] for diagnosis of ME/CFS have had a major international impact on popular perceptions of the disease and on public policies on treating and researching it. | ||
===Media=== | ====Media==== | ||
[[File:DailyTelegraphExercisePositivity.jpg|400px|thumb|right|Daily Telegraph headline suggesting positivity and exercise]] | [[File:DailyTelegraphExercisePositivity.jpg | 400px | thumb | right | Daily Telegraph headline suggesting positivity and exercise]] | ||
On February 27, 2011, when the first PACE trial paper was published, researchers Michael Sharpe and Trudie Chalder held a press conference<ref name="pace2011press1" /><ref name="pace2011press2" /><ref name="pace2011press3" /> to discuss their findings. Chalder stated, | On February 27, 2011, when the first PACE trial paper was published, researchers Michael Sharpe and Trudie Chalder held a press conference<ref name="pace2011press1" /><ref name="pace2011press2" /><ref name="pace2011press3" /> to discuss their findings. Chalder stated, "twice as many people on graded exercise therapy and cognitive behaviour therapy got back to normal."<ref name="Guardian20110218" /> That assertion has been criticized for grossly overstating the study's actual findings.<ref name="Tuller20151021" /><ref name="Tuller20151022" /><ref name="Tuller20151023" /> | ||
The claims made about the study were covered in the UK and international press.<ref name="Reuters20110217" /><ref name="WebMD20110217" /><ref name="BBCUK20110218" /><ref name="CNNnews20110218" /><ref name="MedNewsToday2013" /> For example, The Daily Mail stated, "Fatigued patients who go out and exercise have best hope of recovery",<ref name="DailyMail20110218" /> while The New York Times declared "Psychotherapy Eases Chronic Fatigue Syndrome".<ref name="NYT20110218" /> According to the British Medical Journal's report on the trial, some participants were "cured."<ref name="Tuller20151021" /> | The claims made about the study were covered in the UK and international press.<ref name="Reuters20110217">{{citation | last1 = Reuters | title = Pushing limits can help chronic fatigue patients | date = Feb 17, 2011 | url = http://www.reuters.com/article/us-fatigue-me-idUSTRE71H02320110218}}</ref><ref name="WebMD20110217">{{cite web | last1 = WebMD (US) | title = Study Shows Cognitive Behavioral Therapy, Exercise Are Safe Ways to Treat CFS Symptoms | date = Feb 17, 2011 | url = http://www.webmd.com/chronic-fatigue-syndrome/news/20110217/therapy-exercise-help-chronic-fatigue-syndrome }}</ref><ref name="BBCUK20110218" /><ref name="CNNnews20110218">{{cite news | last1 = CNN News (US) | title = Study supports use of 2 controversial treatments for chronic fatigue | date = Feb 18, 2011 | url = http://edition.cnn.com/2011/HEALTH/02/17/chronic.fatigue/ }}</ref><ref name="MedNewsToday2013">{{citation | last1 = Medical News Today | title = Chronic Fatigue Treatments Lead To Recovery In Trial | date = Feb 1, 2013 | url = http://www.medicalnewstoday.com/articles/255720.php }}</ref> For example, The Daily Mail stated, "Fatigued patients who go out and exercise have best hope of recovery",<ref name="DailyMail20110218" /> while The New York Times declared "Psychotherapy Eases Chronic Fatigue Syndrome".<ref name="NYT20110218" /> According to the British Medical Journal's report on the trial, some participants were "cured."<ref name="Tuller20151021" /> | ||
Many other PACE papers followed, although with relatively little media attention until October 2015, when long-term follow-up results were published in [[The Lancet Psychiatry]].<ref name="pace2015FU" /><ref name="EveningStdUK20151028" /><ref name="IndepIE20151028" /><ref name="Torjesen20151028" /><ref name="DailyMail20151028" /><ref name="FoxNews20150114" /><ref name="Guardian20150114" /> The Daily Telegraph ran a front-page story with the headline, "Exercise and positivity can overcome ME."<ref name="Telegraph20151028" /><ref name="MEAction20151028ptcg" /> The piece stated, "Chronic Fatigue Syndrome is not actually a chronic illness and sufferers can overcome symptoms by increasing exercise and thinking positively, Oxford University has found". The article quoted Professor Sharpe describing [[Myalgic encephalomyelitis|ME]] as a | Many other PACE papers followed, although with relatively little media attention until October 2015, when long-term follow-up results were published in [[The Lancet Psychiatry]].<ref name="pace2015FU" /><ref name="EveningStdUK20151028" /><ref name="IndepIE20151028" /><ref name="Torjesen20151028">{{citation | last1 = Torjesen | first1 = Ingrid | title = Tackling fear about exercise produces long term benefit in chronic fatigue syndrome | journal = The BMJ | date = Oct 28, 2015 | url = http://www.bmj.com/content/351/bmj.h5771 }}</ref><ref name="DailyMail20151028">{{cite news | publisher = Daily Mail | title = ME can be beaten by taking more exercise and positive thinking, landmark study claims | date = Oct 28, 2015 | url = http://www.dailymail.co.uk/health/article-3292782/All-mind-cured-counselling-says-Oxford-professor-claims-sufferers-not-push-recover.html#comments }}</ref><ref name="FoxNews20150114">{{cite news | last1 = FoxNews | title = Helping chronic fatigue patients over fears eases symptoms | date = Jan 14, 2015 | url = http://www.foxnews.com/health/2015/01/14/helping-chronic-fatigue-patients-over-fears-eases-symptoms.html }}</ref><ref name="Guardian20150114" /> The Daily Telegraph ran a front-page story with the headline, "Exercise and positivity can overcome ME."<ref name="Telegraph20151028">{{cite news | publisher = The Telegraph | first = Sarah | last = Knapton | date = Oct 28, 2015 | title = Chronic Fatigue Syndrome sufferers 'can overcome symptoms of ME with positive thinking and exercise | url = http://www.telegraph.co.uk/news/health/11959193/Chronic-Fatigue-Syndrome-sufferers-can-overcome-symptoms-of-ME-with-positive-thinking-and-exercise.html }}</ref><ref name="MEAction20151028ptcg" /> The piece stated, "Chronic Fatigue Syndrome is not actually a chronic illness and sufferers can overcome symptoms by increasing exercise and thinking positively, Oxford University has found". The article quoted Professor Sharpe describing [[Myalgic encephalomyelitis | ME]] as a "self-fulfilling prophesy" that happens when patients live within their limits. The article was altered following public pressure but no formal retraction was made. Science Magazine also published an article in October 2015 along with comments from Sharpe about the growing criticism outwith the patient community from the broader science community.<ref name="ScienceMag20151027" /> | ||
In the UK the [[Science Media Centre]] is a government-funded body that describes its purpose as being to improve science journalism. Its reporting on ME/CFS has been criticized for bias towards a psychological etiology for the disease.<ref name="MEACTUK20110416" /> | In the UK the [[Science Media Centre]] is a government-funded body that describes its purpose as being to improve science journalism. Its reporting on ME/CFS has been criticized for bias towards a psychological etiology for the disease.<ref name="MEACTUK20110416" /> | ||
===Influence on treatment=== | ====Influence on treatment==== | ||
The | The large size of the PACE trial has meant that it has had a substantial impact on the evidence base in ME/CFS. Together with other studies of [[Cognitive behavioral therapy | CBT]] and [[Graded exercise therapy | GET]], it is highly influential in UK clinical policy and that of many other countries, both in terms of healthcare provided by government<ref name="NICEguideCG53" /> and by private medical insurance.<ref name="SwissRe2011" /><ref name="SwissRe2011b" /> The influential [[Cochrane]] exercise therapy review relied significantly on the PACE trial.<ref name="Cochrane-Exercise-2019">{{Cite journal | title = Exercise therapy for chronic fatigue syndrome | date = 2019-10-02 | url = http://doi.wiley.com/10.1002/14651858.CD003200.pub8 | journal = Cochrane Database of Systematic Reviews | volume = 2021 | issue = 3 | last = Larun | first = Lillebeth | last2 = Brurberg | first2 = Kjetil G | last3 = Odgaard-Jensen | first3 = Jan | last4 = Price | first4 = Jonathan R | language = en | editor-last = Cochrane Common Mental Disorders Group | doi = 10.1002/14651858.CD003200.pub8 | pmc = PMC6953363 | pmid = 31577366}}</ref> | ||
In the UK, the NICE guidelines for [[National Health Service | In the UK, the NICE guidelines for [[National Health Service]] (NHS) provided care<ref name="NICEguideCG53" /> recommend CBT and GET for ME/CFS. They were published in 2007, before the PACE trial was conducted, but the evidence was based on a few small trials and was considered "somewhat limited".<ref name="BACME201103" /> The ME Association has asked for the guidelines to be updated to take into account new treatment evidence, noting, "we assume that the guideline surveillance review that took place in March 2011, and which followed publication of the PACE trial in February 2011, simply ‘rubber stamped’ the 2007 NICE guideline recommendations on the basis that the PACE trial had supported the recommendations relating to CBT and GET."<ref name="MEASSUK20131023" /> NICE responded, "we still do not feel that the evidence base is substantially evolving in this area at this time".<ref name="NICEguideCG53static" /> After considerable objections, this decision was reversed and the [[NICE guidelines]] clinical review began in 2017.<ref>{{Cite web | url = https://www.nice.org.uk/guidance/cg53/resources/surveillance-report-2017-chronic-fatigue-syndromemyalgic-encephalomyelitis-or-encephalopathy-diagnosis-and-management-2007-nice-guideline-cg53-4602203537/chapter/Surveillance-decision | title = Surveillance decision {{!}} Chronic fatigue syndrome/myalgic encephalomyelitis (or encephalopathy): diagnosis and management {{!}} Guidance and guidelines | last = National Institute for Clinical Excellence | first = | authorlink = NICE guidelines | date = | website = nice.org.uk | language = en-GB | archive-url = | archive-date = | url-status = | access-date = 2018-10-19}}</ref> | ||
In the US, the | In the US, the Mayo Clinic, Cleveland Clinic, Kaiser Permanente, and numerous key secondary medical education providers, such as UpToDate and WebMD, recommend CBT and GET, using PACE as a reference. CBT and GET were included in the Center for Disease Control's clinical guidelines for CFS, based on the PACE trial evidence.<ref name="CDCguideMECFS" /> | ||
==Criticisms of the study== | ===Criticisms of the study=== | ||
===Selection of patients=== | ====Selection of patients==== | ||
The PACE trial used the [[Oxford criteria]] for diagnosis. Many patients and specialist clinicians consider them overly broad,<ref name="EgelandT2015" /><ref name="JasonLA2016casedef" /> and the National Institutes of Health 2015 P2P report<ref name="NIHP2PFIN" /> on ME/CFS recommended that the Oxford definition be retired for this reason. | The PACE trial used the [[Oxford criteria]] for diagnosis. Many patients and specialist clinicians consider them overly broad,<ref name="EgelandT2015" /><ref name="JasonLA2016casedef" /> and the National Institutes of Health 2015 P2P report<ref name="NIHP2PFIN" /> on ME/CFS recommended that the Oxford definition be retired for this reason. | ||
===Changes in criteria for effectiveness and recovery=== | ====Changes in criteria for effectiveness and recovery==== | ||
[[File:PACEthreshold.jpg|thumb|right|414px|PACE recovery thresholds - author confusion. <br>Image: Senseaboutscienceusa.org]] | [[File:PACEthreshold.jpg | thumb | right | 414px | PACE recovery thresholds - author confusion. <br>Image: Senseaboutscienceusa.org]] | ||
The authors abandoned their protocol-specified main outcome and recovery analyses partway through the trial and replaced them with others.<ref name="pace2011a" /><ref name="pace2013a" /> They have defended the changes, noting, "All these changes were made before any outcome data were analyzed (i.e. they were pre-specified), and were all approved by the independent [[PACE Trial Steering Committee]] and [[Data Monitoring and Ethics Committee]]."<ref name="Tuller20151030a" /> However, 42 scientists, in an open letter to | The authors abandoned their protocol-specified main outcome and recovery analyses partway through the trial and replaced them with others.<ref name="pace2011a" /><ref name="pace2013a" /> They have defended the changes, noting, "All these changes were made before any outcome data were analyzed (i.e. they were pre-specified), and were all approved by the independent [[PACE Trial Steering Committee]] and [[Data Monitoring and Ethics Committee]]."<ref name="Tuller20151030a" /> However, 42 scientists, in an [[open letter to the Lancet]], stated that the changes were of "of particular concern in an unblinded trial like PACE, in which outcome trends are often apparent long before outcome data are seen. The investigators provided no sensitivity analyses to assess the impact of the changes and have refused requests to provide the results per the methods outlined in their protocol."<ref name="openletrLANCET2" /> | ||
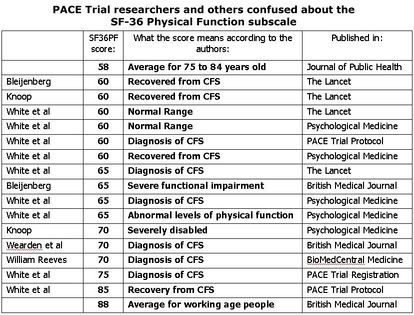
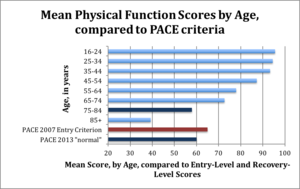
Most notably, the authors introduced post-hoc "normal ranges" for fatigue and physical function.<ref name="pace2011a" /> These ranges have been heavily criticised for having thresholds so low that patients could worsen from trial entry and yet be within these normal ranges. The "normal range" for physical function (measured on the [[SF-36]] 100-point scale) was 60 and above, even though patients had to score 65 or lower to enter the trial. A score of 60 is close to the mean physical function score (57) of patients with Class II coronary heart failure.<ref name="Juenger2012" /> | Most notably, the authors introduced post-hoc "normal ranges" for fatigue and physical function.<ref name="pace2011a" /> These ranges have been heavily criticised for having thresholds so low that patients could worsen from trial entry and yet be within these normal ranges. The "normal range" for physical function (measured on the [[SF-36]] 100-point scale) was 60 and above, even though patients had to score 65 or lower to enter the trial. A score of 60 is close to the mean physical function score (57) of patients with Class II coronary heart failure.<ref name="Juenger2012" /> | ||
[[File:PACE-SF36-stats.org.png|thumb|right|300px|PACE recovery thresholds - much lower than for healthy people of similar age. Image: Senseaboutscienceusa.org]]<blockquote>"The average age of participants in the PACE trial is about 39 years old; normative data suggest that people in this age group should have SF-36 scores of about 93. Yet the new 2013 “normal” is a score of 60."<ref name="GoldinR20160321" /></blockquote> | [[File:PACE-SF36-stats.org.png | thumb | right | 300px | PACE recovery thresholds - much lower than for healthy people of similar age. Image: Senseaboutscienceusa.org]]<blockquote>"The average age of participants in the PACE trial is about 39 years old; normative data suggest that people in this age group should have SF-36 scores of about 93. Yet the new 2013 “normal” is a score of 60."<ref name="GoldinR20160321" /></blockquote> | ||
The PACE authors used the "normal ranges", in conjunction with other thresholds, to define clinical effectiveness in the Lancet<ref name="MEAction20151028lancet" /> paper and recovery rates in a later paper in | The PACE authors used the "normal ranges", in conjunction with other thresholds, to define clinical effectiveness in the Lancet<ref name="MEAction20151028lancet" /> paper and recovery rates in a later paper in the Journal of Psychological Medicine.<ref name="MEAction20151028psymed" /> | ||
All Freedom of Information requests to the authors for the main outcome and recovery results according to the protocol-specified analyses, or for the underlying data so that others could conduct the analyses, have been refused.<ref name="FOI20131029C" /><ref name="FOI20140324M" /><ref name="FOI20150629GM" /><ref name="FOI20160309AS" /> | All Freedom of Information requests to the authors for the main outcome and recovery results according to the protocol-specified analyses, or for the underlying data so that others could conduct the analyses, have been refused.<ref name="FOI20131029C" /><ref name="FOI20140324M" /><ref name="FOI20150629GM" /><ref name="FOI20160309AS" /> | ||
===Use of subjective main outcome measures=== | ====Use of subjective main outcome measures==== | ||
The study has been criticised for having subjective primary analyses in an unblinded trial.<ref name="EdwardsJC20150118" /> Subjective measures are known to be susceptible to bias, as can arise from expectations and social pressure. The CBT and GET groups, but not the others, were told that there was nothing to stop them from recovering if they gradually increased their activity, and critics have argued that these differential expectations could have inflated their self-assessments.<ref name="openletrLANCET1" /><ref name="EdwardsJC20151101" /> | The study has been criticised for having subjective primary analyses in an unblinded trial.<ref name="EdwardsJC20150118" /> Subjective measures are known to be susceptible to bias, as can arise from expectations and social pressure. The CBT and GET groups, but not the others, were told that there was nothing to stop them from recovering if they gradually increased their activity, and critics have argued that these differential expectations could have inflated their self-assessments.<ref name="openletrLANCET1" /><ref name="EdwardsJC20151101" /> | ||
===Conflicts of interest and lack of informed consent=== | ====Conflicts of interest and lack of informed consent==== | ||
The forty-two scientists and clinicians who wrote an [[open letter to the Lancet]] complaining about the PACE trial criticized the study authors' failure to disclose a potential conflict of interest to trial participants.<ref name="openletrLANCET2" /> They wrote: | The forty-two scientists and clinicians who wrote an [[open letter to the Lancet]] complaining about the PACE trial criticized the study authors' failure to disclose a potential conflict of interest to trial participants.<ref name="openletrLANCET2" /> They wrote: | ||
| Line 89: | Line 88: | ||
<blockquote>"The investigators violated their promise in the PACE protocol to adhere to the Declaration of Helsinki, which mandates that prospective participants be 'adequately informed' about researchers’ “possible conflicts of interest.” The main investigators have had financial and consulting relationships with disability insurance companies, advising them that rehabilitative therapies like those tested in PACE could help ME/CFS claimants get off benefits and back to work. They disclosed these insurance industry links in The Lancet but did not inform trial participants, contrary to their protocol commitment. This serious ethical breach raises concerns about whether the consent obtained from the 641 trial participants is legitimate."</blockquote> | <blockquote>"The investigators violated their promise in the PACE protocol to adhere to the Declaration of Helsinki, which mandates that prospective participants be 'adequately informed' about researchers’ “possible conflicts of interest.” The main investigators have had financial and consulting relationships with disability insurance companies, advising them that rehabilitative therapies like those tested in PACE could help ME/CFS claimants get off benefits and back to work. They disclosed these insurance industry links in The Lancet but did not inform trial participants, contrary to their protocol commitment. This serious ethical breach raises concerns about whether the consent obtained from the 641 trial participants is legitimate."</blockquote> | ||
===Newsletter to participants=== | ====Newsletter to participants==== | ||
The investigators published newsletters for participants<ref name="pacenwsltr1" /><ref name="pacenwsltr2" /><ref name="pacenwsltr3" /><ref name="pacenwsltr4" /> while the trial was still underway. Critics have said that the material in the third newsletter<ref name="pacenwsltr3" /> could have influenced patients' self-reported outcomes. It included a number of positive testimonials from patients in the trial, but without naming their therapies. The PACE authors have argued that this meant that there would be no bias in favour of CBT and GET<ref name="Tuller20151030a" /> but Professor James Coyne has dismissed the idea that bias would be expected to affect all four groups equally. | The investigators published newsletters for participants<ref name="pacenwsltr1">{{citation | last1 = PACE Trial Management Group | authorlink1 = PACE Trial Management Group | title = PACE trial participants' newsletter #1 | date = Jun 2006 | url = https://www.qmul.ac.uk/wolfson/media/wolfson/current-projects/participantsnewsletter1.pdf}}</ref><ref name="pacenwsltr2">{{citation | last1 = PACE Trial Management Group | authorlink1 = PACE Trial Management Group | title = PACE trial participants' newsletter #2 | date = Mar 2007 | url = https://www.qmul.ac.uk/wolfson/media/wolfson/current-projects/participantsnewsletter2.pdf}}</ref><ref name="pacenwsltr3">{{citation | last1 = PACE Trial Management Group | authorlink1 = PACE Trial Management Group | title = PACE trial participants' newsletter #3 | date = Dec 2008 | url = https://www.qmul.ac.uk/wolfson/media/wolfson/current-projects/participantsnewsletter3.pdf}}</ref><ref name="pacenwsltr4">{{citation | last1 = PACE Trial Management Group | authorlink1 = PACE Trial Management Group | title = PACE trial participants' newsletter #4 | date = Feb 2011 | url = https://www.qmul.ac.uk/wolfson/media/wolfson/current-projects/participantsnewsletter4.pdf }}</ref> while the trial was still underway. Critics have said that the material in the third newsletter<ref name="pacenwsltr3" /> could have influenced patients' self-reported outcomes. It included a number of positive testimonials from patients in the trial, but without naming their therapies. The PACE authors have argued that this meant that there would be no bias in favour of CBT and GET<ref name="Tuller20151030a" /> but Professor James Coyne has dismissed the idea that bias would be expected to affect all four groups equally.<ref name="Coyne20151029mb" /> | ||
The newsletter did, however, announce that the new NICE guidelines, "based on the best available evidence... recommended therapies [that] include Cognitive Behavioural Therapy, Graded Exercise Therapy and Activity Management." There was no explanation of what "Activity Management" was: no group had that title in the PACE trial. Dr. Bruce Levin, a professor of biostatistics at Columbia University and an expert in clinical trial design, said, | The newsletter did, however, announce that the new NICE guidelines, "based on the best available evidence... recommended therapies [that] include Cognitive Behavioural Therapy, Graded Exercise Therapy and Activity Management." There was no explanation of what "Activity Management" was: no group had that title in the PACE trial. Dr. Bruce Levin, a professor of biostatistics at Columbia University and an expert in clinical trial design, said, "To let participants know that interventions have been selected by a government committee 'based on the best available evidence' strikes me as the height of clinical trial amateurism".<ref name="Tuller20151021" /> | ||
The newsletter also contained a less than positive assessment of research on the possibility of an infectious component of ME/CFS, including research by [[Jose Montoya]] on [[herpesvirus]]es and by [[John Chia]] on [[enterovirus]]es. The newsletter said of Dr Chia's work, for example, "The laboratory work looked convincing, but many patients had significant gastro-intestinal symptoms and even signs, casting some doubt on the diagnoses of CFS being the correct or sole diagnosis in these patients." It is possible that this negative view of evidence of an ongoing infection would have made the rationale for APT appear less plausible and that for CBT and GET more plausible, thus biasing the participants. | The newsletter also contained a less than positive assessment of research on the possibility of an infectious component of ME/CFS, including research by [[Jose Montoya]] on [[herpesvirus]]es and by [[John Chia]] on [[enterovirus]]es. The newsletter said of Dr Chia's work, for example, "The laboratory work looked convincing, but many patients had significant gastro-intestinal symptoms and even signs, casting some doubt on the diagnoses of CFS being the correct or sole diagnosis in these patients." It is possible that this negative view of evidence of an ongoing infection would have made the rationale for APT appear less plausible and that for CBT and GET more plausible, thus biasing the participants. | ||
An account of another study, in contrast, gave a positive assessment of CBT, saying "cognitive behaviour therapy was associated with an increase in grey matter of the brain and this increase was associated with improved cognitive function". | An account of another study, in contrast, gave a positive assessment of CBT, saying "cognitive behaviour therapy was associated with an increase in grey matter of the brain and this increase was associated with improved cognitive function". | ||
===Risks and side effects=== | ====Risks and side effects==== | ||
A survey<ref name="MEASSUK20150529survey" /> conducted by the [[ME Association]] in 2012 showed that 74% of patients had their symptoms worsen after a course of GET. In contrast, the PACE trial found no apparently meaningful difference in rates of adverse events between the four trial groups,<ref name="pace2014AE" /> suggesting that APT, CBT and GET added no risk to SMC alone (since all four groups received SMC). However, critics have questioned whether patients actually increased their activity sufficiently in the CBT and GET groups to trigger many serious adverse events:<ref name="Kindlon2011" /><ref name="Tuller20160107" /> the lack of improvement in the step-fitness test in all groups indicates that this is distinctly possible.<ref name="pace2015SMA" /> | A survey<ref name="MEASSUK20150529survey" /> conducted by the [[ME Association]] in 2012 showed that 74% of patients had their symptoms worsen after a course of GET. In contrast, the PACE trial found no apparently meaningful difference in rates of adverse events between the four trial groups,<ref name="pace2014AE" /> suggesting that APT, CBT and GET added no risk to SMC alone (since all four groups received SMC). However, critics have questioned whether patients actually increased their activity sufficiently in the CBT and GET groups to trigger many serious adverse events:<ref name="Kindlon2011" /><ref name="Tuller20160107" /> the lack of improvement in the step-fitness test in all groups indicates that this is distinctly possible.<ref name="pace2015SMA" /> | ||
===Analysis by "citizen-scientists" - ME Sufferers and Charities=== | |||
Numerous ME sufferers and other interested parties have produced critiques of the PACE trial including statistical analyses and trial methodology. | |||
'''[[Robert Courtney]]''' | |||
Mr Courtney has written a number of published letters in the medical journals, criticising PACE. | Mr Courtney has written a number of published letters in the medical journals, criticising PACE. | ||
<blockquote>"Chalder and colleagues acknowledge that the trial outcomes do not support the hypothetical [[deconditioning]] model of GET for chronic fatigue syndrome".<ref name="CourtneyRob201504lanpsy" /></blockquote> | <blockquote>"Chalder and colleagues acknowledge that the trial outcomes do not support the hypothetical [[deconditioning]] model of GET for chronic fatigue syndrome".<ref name="CourtneyRob201504lanpsy" /></blockquote> | ||
'''[[Peter Kemp]]''' | |||
[[Peter Kemp]] has written a detailed thirty page critique of the study split into ten sections called the [https://docs.google.com/document/d/1AqCB7ZywQGYyR8fz5QICu4PDHvJt_RXjebNJah_Dz7U/view#heading = h.d4e0wlbznjk1 'PACE Trial Analysis]'.<ref name="KempPet201602" /> | |||
[[ | '''[[Angela Kennedy]]''' | ||
[[Angela Kennedy]] has made specific critiques of PACE regarding the following areas:<ref name="KennedyAng20160210" /> | [[Angela Kennedy]] has made specific critiques of PACE regarding the following areas:<ref name="KennedyAng20160210" /> | ||
| Line 128: | Line 128: | ||
# The instability of 'specialist medical care' as a treatment category, and the lack of any sound category of 'control' group. | # The instability of 'specialist medical care' as a treatment category, and the lack of any sound category of 'control' group. | ||
'''[[Frank Twisk]]''' | |||
Dutch patient Frank Twisk of the ME-de-patiënten Foundation has also published criticism of the PACE trial.<ref name="Twisk20160118" /> | Dutch patient Frank Twisk of the ME-de-patiënten Foundation has also published criticism of the PACE trial.<ref name="Twisk20160118" /> | ||
<blockquote>"The PACE trial investigated the effects of CBT and GET in chronic fatigue, as defined by the Oxford criteria, not in chronic fatigue syndrome, let alone myalgic encephalomyelitis".</blockquote> | <blockquote>"The PACE trial investigated the effects of CBT and GET in chronic fatigue, as defined by the Oxford criteria, not in chronic fatigue syndrome, let alone myalgic encephalomyelitis".</blockquote> | ||
<blockquote>"[T]he positive effect of CBT and GET in subjective measures, fatigue and physical functioning, cannot be qualified as sufficient. Mean [[Short Form 36-Item Health Survey|short form-36]] physical functioning scores in the CBT group (62·2) and the GET group (59·8) at follow-up were below the inclusion cutoff score for the PACE trial (≤65)<sup>3</sup> and far below the objective for recovery as defined in the PACE protocol (≥85)."</blockquote> | <blockquote>"[T]he positive effect of CBT and GET in subjective measures, fatigue and physical functioning, cannot be qualified as sufficient. Mean [[Short Form 36-Item Health Survey | short form-36]] physical functioning scores in the CBT group (62·2) and the GET group (59·8) at follow-up were below the inclusion cutoff score for the PACE trial (≤65)<sup>3</sup> and far below the objective for recovery as defined in the PACE protocol (≥85)."</blockquote> | ||
<blockquote>"The vast majority of patients improved subjectively by specialist medical care and APT to the same level as by CBT and GET, without any additional therapies, including CBT and GET, or by other therapies."</blockquote> | <blockquote>"The vast majority of patients improved subjectively by specialist medical care and APT to the same level as by CBT and GET, without any additional therapies, including CBT and GET, or by other therapies."</blockquote> | ||
| Line 139: | Line 140: | ||
<blockquote>"[L]ooking at subjective outcomes at follow-up and objective outcomes in earlier studies, such as physical fitness, return to employment, social welfare benefits, and health-care usage, CBT and GET, like specialist medical care and APT, cannot be qualified as effective".</blockquote> | <blockquote>"[L]ooking at subjective outcomes at follow-up and objective outcomes in earlier studies, such as physical fitness, return to employment, social welfare benefits, and health-care usage, CBT and GET, like specialist medical care and APT, cannot be qualified as effective".</blockquote> | ||
'''[[Tom Kindlon]]''' | |||
[[Tom Kindlon]] a patient and Vice-Chairman of the [[Irish ME/CFS Association]]. He has published extensive criticism of the PACE trial. In 2011 he published a paper on harms associated with [[Graded exercise therapy]].<ref name="Kindlon2011" /> | [[Tom Kindlon]] a patient and Vice-Chairman of the [[Irish ME/CFS Association]]. He has published extensive criticism of the PACE trial. In 2011 he published a paper on harms associated with [[Graded exercise therapy]].<ref name="Kindlon2011" /> | ||
| Line 147: | Line 148: | ||
Among many other published letters that have been critical of the trial, many are from people who have identified themselves as patients. For example, most of the letters published by The Lancet criticising the 2011 PACE paper were from patients or representatives of patients' groups.<ref name="Mitchell20110517" /><ref name="FeehanSar20110517lancet" /><ref name="Giakoumakis20110517lancet" /> | Among many other published letters that have been critical of the trial, many are from people who have identified themselves as patients. For example, most of the letters published by The Lancet criticising the 2011 PACE paper were from patients or representatives of patients' groups.<ref name="Mitchell20110517" /><ref name="FeehanSar20110517lancet" /><ref name="Giakoumakis20110517lancet" /> | ||
'''ME Analysis: Evaluating the results of the PACE Trial study''' | |||
[[Janelle Wiley]] and [[Graham McPhee]] also collaborated with others from Phoenix Rising to create [https://www.s4me.info/evaluatingpace/homepageanim.html 'ME Analysis: Evaluating the results of the PACE Trial' website] summarising their critique of the trial. | [[Janelle Wiley]] and [[Graham McPhee]] also collaborated with others from Phoenix Rising to create [https://www.s4me.info/evaluatingpace/homepageanim.html 'ME Analysis: Evaluating the results of the PACE Trial' website] summarising their critique of the trial. | ||
| Line 153: | Line 154: | ||
It examined the flaws with the PACE trial and came up with 10 conclusions. The 'ME Analysis: Evaluating the results of the PACE Trial' is presented in the form of [https://www.s4me.info/evaluatingpace/summary.html 10 conclusions]. Clicking on each conclusion will lead to a short summary, and clicking on the links underneath each summary will, if you wish, lead you ever deeper into our analysis. | It examined the flaws with the PACE trial and came up with 10 conclusions. The 'ME Analysis: Evaluating the results of the PACE Trial' is presented in the form of [https://www.s4me.info/evaluatingpace/summary.html 10 conclusions]. Clicking on each conclusion will lead to a short summary, and clicking on the links underneath each summary will, if you wish, lead you ever deeper into our analysis. | ||
'''ME Analysis videos by Phoenix Rising''' | |||
Members of [[Phoenix Rising]] including [[Graham McPhee]] and [[Tom Kindlon]] have collaborated on a set of explanatory videos about flaws in PACE's statistical analyses on YouTube: | Members of [[Phoenix Rising]] including [[Graham McPhee]] and [[Tom Kindlon]] have collaborated on a set of explanatory videos about flaws in PACE's statistical analyses on YouTube: | ||
| Line 164: | Line 165: | ||
* [https://www.youtube.com/watch?v=d_7J5ELjArU Video 7: How's That Recovery?] | * [https://www.youtube.com/watch?v=d_7J5ELjArU Video 7: How's That Recovery?] | ||
'''ME Charities criticism and complaints''' | |||
Charities have criticised it including [[Invest in ME Research]].<ref name="InvIME20151114" /> [[Jane Colby]] of [[Tymes Trust]] wrote a letter to the Guardian.<ref name="Guardian20110224truth" /> The ME Association have also criticised the PACE trial. | |||
'''MEAction''' | |||
[[MEAction]] submitted a petition to retract the PACE trial that received over 12,000 signatures.<ref>{{Cite web | url = https://www.meaction.net/reach/press-releases/press-release-12000-signature-pace-petition-delivered-to-the-lancet/ | title = PRESS RELEASE: 12,000 SIGNATURE PACE PETITION DELIVERED TO THE LANCET | date = 2015 | last = #MEAction | first = | authorlink = The MEAction Network | website = MEAction | archive-url = | archive-date = | url-status = | access-date = Jun 10, 2020}}</ref> In addition, #MEAction published a [http://www.meaction.net/pace-trial/ 'PACE Trial overview'] of the PACE trial and its flaws, produced by patients.<ref name="MEACTION20151028pto" /> MEAction have also produced a factsheet of [http://www.meaction.net/wp-content/uploads/2015/05/MEAction–patient-view-of-the-PACE-Trial-Controversy.pdf 'Why ME patients are critical of the PACE trial'] which addresses three major myths which have been created by the PACE trial investigators and the harms that these myths have caused. The myths busted included: | |||
MYTH: 1 'The controversy is fueled by a vocal minority of "vociferous" ME militants on the internet, | |||
MYTH 2: M E sufferers oppose GET because they are [[kinesphobia | afraid of exercise]]. | |||
MYTH 3: ME sufferers oppose CBT because they are afraid of the stigma of [[mental illness]].<ref name="MEACTION201505pvpt" /> | |||
'''Briefing document from a [[Science for ME]] working group''' | |||
Consisting of patients including [[Tom Kindlon]], [[Sean Kirby]] and [[Graham McPhee]], this working group has produced a document to concisely explain the flaws in the PACE trial. | Consisting of patients including [[Tom Kindlon]], [[Sean Kirby]] and [[Graham McPhee]], this working group has produced a document to concisely explain the flaws in the PACE trial. | ||
| Line 177: | Line 185: | ||
* [https://s4me.info/threads/science-for-me-pace-briefing-document.3140/ The PACE Trial Controversy: A Summary] | * [https://s4me.info/threads/science-for-me-pace-briefing-document.3140/ The PACE Trial Controversy: A Summary] | ||
'''ME Analysis videos (2018)''' | |||
[[Graham McPhee]] has created a set of three videos looking again at the PACE trial. | [[Graham McPhee]] has created a set of three videos looking again at the PACE trial. | ||
* [https:// | * [https://www.youtube.com/watch?v=RqpHc_Y7awQ "The PACE study claimed that CBT and graded exercise helped (or even cured) people with ME. This easy video explains its main faults."] | ||
Others including Jessica Kellgren-Fozard, a vlogger and TV producer with a large social media following, created a video in May 2018 called 'Have you been misled...? // What is PACE? // Medical Scandal' which had been viewed over 35,000 times <ref>https://www.youtube.com/watch?v=7NKsbjU89Zk </ref> | Others including Jessica Kellgren-Fozard, a vlogger and TV producer with a large social media following, created a video in May 2018 called 'Have you been misled...? // What is PACE? // Medical Scandal' which had been viewed over 35,000 times.<ref>{{Cite web | access-date = 2010-10-06 | title = Have you been misled...? // What is PACE? // Medical Scandal | first = Jessica | last = Kellgren-Fozard | url = https://www.youtube.com/watch?v=7NKsbjU89Zk | website = YouTube | date = May 7, 2018 }}</ref> | ||
==Controversy== | ===Controversy=== | ||
The PACE Trial has been heavily criticised by patient groups and some researchers and science journalists for a number of methodological problems since its publication. <ref name="Tuller20151021" /><ref name="Tuller20151022" /><ref name="Tuller20151023" /><ref name="Tuller20151030a" /><ref name="Tuller20151030b" /><ref name="Tuller20151104" /><ref name="Tuller20151109" /><ref name="Tuller20151117" /><ref name="Tuller20160104" /> | The PACE Trial has been heavily criticised by patient groups and some researchers and science journalists for a number of methodological problems since its publication. <ref name="Tuller20151021" /><ref name="Tuller20151022" /><ref name="Tuller20151023" /><ref name="Tuller20151030a" /><ref name="Tuller20151030b" /><ref name="Tuller20151104" /><ref name="Tuller20151109" /><ref name="Tuller20151117" /><ref name="Tuller20160104" /> | ||
=== Prof Malcolm Hooper's complaints === | ====Prof Malcolm Hooper's complaints==== | ||
Prof [[Malcolm Hooper]] and [[Margaret Williams]] have followed the PACE trial from its inception in 2004 and provided salutary warnings about the possible issues and problems with the conduct of the trial due to their previous knowledge of the principal investigators research.<ref name="HoopWill20040620" /><ref name="HoopWill20091217" /> | Prof [[Malcolm Hooper]] and [[Margaret Williams]] have followed the PACE trial from its inception in 2004 and provided salutary warnings about the possible issues and problems with the conduct of the trial due to their previous knowledge of the principal investigators research.<ref name="HoopWill20040620" /><ref name="HoopWill20091217" /> | ||
They then published a 400 page critique of the PACE trial in February 2010 'Magical Medicine: How to make a disease disappear'.<ref name="HoopWill201002MM" /> In February 2011 upon publication of the PACE trial findings, Prof Hooper submitted a comprehensive complaint to the editor of the Lancet<ref name="HoopWill201103" /> and a further detailed response<ref name="HoopWill20110528" />. | They then published a 400 page critique of the PACE trial in February 2010 'Magical Medicine: How to make a disease disappear'.<ref name="HoopWill201002MM">{{cite web | last1 = Hooper | first1 = Malcolm | authorlink1 = Malcolm Hooper | title = Magical Medicine: How to Make a Disease Disappear | date = Feb 2010 | url = http://www.margaretwilliams.me/2010/magical-medicine_hooper_feb2010.pdf }}</ref> In February 2011 upon publication of the PACE trial findings, Prof Hooper submitted a comprehensive complaint to the editor of the Lancet<ref name="HoopWill201103" /> and a further detailed response.<ref name="HoopWill20110528" /> | ||
Prof Hooper et al in 2011 published further concerns about the PACE trial<ref name="HoopWill20110604" /> and have also examined the role of the Science Media Centre and the insurance industry with the PACE trial.<ref name="HoopWill201309a" /> The Key Concerns about the PACE trial were also published in 2013 by MEActionUK.<ref name="HoopWill201309b" /> Prof Hooper has published a summary of the key dates and chronology of the trial since 2004.<ref name="HoopWill20151114" /> | |||
====Investigation by public health journalist and academic, Dr. David Tuller==== | |||
Renewed interest in the trial came in October 2015 with public health expert and investigative journalist Dr. [[David Tuller]]'s investigative "Trial by Error: The Troubling Case of the PACE Chronic Fatigue Syndrome Study" publication on Virology Blog which gave a detailed analysis of PACE's methodological problems. Dr. Tuller continues to publish articles criticizing different aspects of the trial.<ref name="viroblogMECFS" /> Some of the main articles for the "Trial by Error" series on the PACE trial are listed below: | |||
*Oct 21, 2015 [http://www.virology.ws/2015/10/21/trial-by-error-i/ "Trial by Error: The Troubling Case of the PACE Chronic Fatigue Syndrome Study"] | |||
*Oct 22, 2015 [http://www.virology.ws/2015/10/22/trial-by-error-ii/ "Trial by Error: The Troubling Case of the PACE Chronic Fatigue Syndrome Study (second instalment)"] | |||
*Oct 23, 2015 [http://www.virology.ws/2015/10/23/trial-by-error-iii/ "Trial by Error: The Troubling Case of the PACE Chronic Fatigue Syndrome Study (final installment)"] | |||
*Nov 13, 2015 [http://www.virology.ws/2015/11/13/an-open-letter-to-dr-richard-horton-and-the-lancet/ "An open letter to Dr. Richard Horton and The Lancet"] | |||
*Jan 7, 2016 [http://www.virology.ws/2016/01/07/trial-by-error-continued-did-the-pace-trial-really-prove-that-graded-exercise-is-safe/ Trial By Error, Continued: Did the PACE Trial Really Prove that Graded Exercise Is Safe?] (with [[Julie Rehmeyer]]) | |||
For a longer list of the main articles on the PACE trial see: | |||
* [[Trial By Error#Main Trial By Error articles on the PACE trial | Main Trial By Error articles on the PACE trial]] | |||
The PACE trial authors, with the exception of their response to Virology on October 30, 2015, have refused to respond to or engage with David Tuller about these concerns; Tuller made requests for comment in 2015 and 2016. Similarly, Richard Horton, editor of the ''Lancet'' has refused to respond to Tuller about these concerns or about the retraction of the PACE trial publication. Sir [[Simon Wessely]] on behalf of the PACE trial principal investigators did publish an article in November 2015 [https://www.nationalelfservice.net/other-health-conditions/chronic-fatigue-syndrome/the-pace-trial-for-chronic-fatigue-syndrome-choppy-seas-but-a-prosperous-voyage/ The PACE trial for chronic fatigue syndrome: choppy seas but a prosperous voyage] referring to the growing concerns over the PACE trial. This was published in a blog website called National Elf Service run by the Mental Elf, in which he used an analogy of the clinical trial as an ocean liner crossing the Atlantic.<ref name="paceWesselyTitanic" /> | The PACE trial authors, with the exception of their response to Virology on October 30, 2015, have refused to respond to or engage with David Tuller about these concerns; Tuller made requests for comment in 2015 and 2016. Similarly, Richard Horton, editor of the ''Lancet'' has refused to respond to Tuller about these concerns or about the retraction of the PACE trial publication. Sir [[Simon Wessely]] on behalf of the PACE trial principal investigators did publish an article in November 2015 [https://www.nationalelfservice.net/other-health-conditions/chronic-fatigue-syndrome/the-pace-trial-for-chronic-fatigue-syndrome-choppy-seas-but-a-prosperous-voyage/ The PACE trial for chronic fatigue syndrome: choppy seas but a prosperous voyage] referring to the growing concerns over the PACE trial. This was published in a blog website called National Elf Service run by the Mental Elf, in which he used an analogy of the clinical trial as an ocean liner crossing the Atlantic.<ref name="paceWesselyTitanic" /> | ||
=== Further criticism from scientific community | ====Further criticism from scientific community==== | ||
Dr [[James Coyne]], Professor of Health Psychology, at the | Dr [[James Coyne]], Professor of Health Psychology, at the University Medical Centre Gronigen]] (UMCG), published on PLoS One Blog on 29 October 2015 "Uninterpretable: Fatal flaws in PACE Chronic Fatigue Syndrome follow-up study".<ref name="Coyne20151029mb" /> He gave a talk at Edinburgh University in November 2015 criticising the PACE trial.<ref name="Coyne20151116-v1" /><ref name="Coyne20151116-v2" /><ref name="Coyne20151116-v3" /><ref name="Coyne20151116-ss" /> He spoke again about the PACE study in Belfast in February 2016 where he described it as "a wasteful trainwreck of a study".<ref name="Coyne20160208-v" /><ref name="Coyne20160208-ss" /> Professor Coyne has also questioned whether the PACE trial paper could ever have been properly peer-reviewed, given the large number of study authors and the small world of British science.<ref name="Coyne20151125" /> He has continued to critique the PACE trial.<ref name="CoyneMECFSIndex" /> | ||
Dr [[Keith Laws]], Professor of Neuropsychology at the | Dr [[Keith Laws]], Professor of Neuropsychology at the University of Hertfordshire has also criticised the PACE trial in a number of blogs on his website in November 2015.<ref name="LawsKth201511ptah">{{cite web | last1 = Laws | first1 = Keith | authorlink1 = Keith Laws | title = PACE - Thoughts about Holes | url = http://keithsneuroblog.blogspot.co.uk/2015/11/pace-thoughts-about-holes.html | website = LawsDystopia Blog | date = Nov 1, 2015}}</ref><ref name="LawsKth201511sfs">{{cite web | last1 = Laws | first1 = Keith | authorlink1 = Keith Laws | title = PACE - Song for the Siren | url = http://keithsneuroblog.blogspot.co.uk/2015/11/song-for-siren.html | website = LawsDystopia Blog | date = Nov 6, 2015 }}</ref> He also co-authored a letter in the ''Lancet Psychiatry'' in 2016 with Dr. Coyne "Results of the PACE follow-up study are uninterpretable".<ref name="CoyneLaws20160118" /> | ||
Science journalist [[Julie Rehmeyer]] published an article in | Science journalist [[Julie Rehmeyer]] published an article in ''Slate'' magazine in November 2015 called "Hope for Chronic Fatigue Syndrome: The debate over this mysterious disease is suddenly shifting" and contrasted the hope that sufferers had with research being conducted in the US and the UK researchers involved with PACE trial.<ref name="Rehmeyer20151113" /> Julie Rehmeyer has continued to criticise the PACE trial with further publications and in conferences.<ref name="NYTimes20170318" /><ref name="<ref name="Rehmeyer20160921" /><ref name="NYTimes20170318">{{Cite news | first = Julie | last = Rehmeyer | authorlink = Julie Rehmeyer | first2 = David | last2 = Tuller | author-link2 = David Tuller | date = Mar 18, 2017 | publisher = The New York Times, Sunday Review | url = https://www.nytimes.com/2017/03/18/opinion/sunday/getting-it-wrong-on-chronic-fatigue-syndrome.html | title = Getting It Wrong on Chronic Fatigue Syndrome}}</ref> | ||
Lucy Bailey, a former editor of the ''Lancet'', analysed the PACE trial and provided her comments on the controversy, concluding, "Psychiatrists need to understand that their presence anywhere near this condition is now toxic, and maybe they need to take a step back".<ref name="BaileyL201601" /> Bailey also wrote a letter to the Lancet Infectious Disease called "A case for Retraction?" and has questioned the reasons why [[actigraphy]] was dropped late in the trial.<ref name="BaileyL201710" /> <ref>https://lucibee.wordpress.com/2018/05/09/pace-trial-whatever-happened-to-actigraphy/</ref> | Lucy Bailey, a former editor of the ''Lancet'', analysed the PACE trial and provided her comments on the controversy, concluding, "Psychiatrists need to understand that their presence anywhere near this condition is now toxic, and maybe they need to take a step back".<ref name="BaileyL201601" /> Bailey also wrote a letter to the Lancet Infectious Disease called "A case for Retraction?" and has questioned the reasons why [[actigraphy]] was dropped late in the trial.<ref name="BaileyL201710" /><ref name="BaileyActigraphy">{{cite web | last1 = Bailey | first1 = Lucy | authorlink1 = Lucy Bailey | title = PACE trial: Whatever happened to actigraphy? | date = May 9, 2018 | website = Lucibee's Blog | url = | ||
https://lucibee.wordpress.com/2018/05/09/pace-trial-whatever-happened-to-actigraphy/ }}</ref> | |||
Dr [[Mark Vink]], a family physician in the [[Netherlands]], published in the | Dr [[Mark Vink]], a family physician in the [[Netherlands]], published in the ''Journal of Neurology and Neurobiology'' in March 2016 that "[t]he PACE Trial Invalidates the Use of Cognitive Behavioral and Graded Exercise Therapy in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome".<ref name="VinkM2016" /> | ||
Prof [[Jonathan Edwards]] has declared that "PACE is valueless for one reason: the combination of lack of blinding of treatments and choice of subjective primary endpoint. Neither of these alone need be a fatal design flaw but the combination is". He also stated "the authors have not been meticulous in trying to avoid bias that might arise. On the contrary they appear to have acted in ways more or less guaranteed to maximise bias."<ref name="EdwardsJC20151101" /> | Prof [[Jonathan Edwards]] has declared that "PACE is valueless for one reason: the combination of lack of blinding of treatments and choice of subjective primary endpoint. Neither of these alone need be a fatal design flaw but the combination is". He also stated "the authors have not been meticulous in trying to avoid bias that might arise. On the contrary they appear to have acted in ways more or less guaranteed to [[research bias in ME/CFS | maximise bias]]."<ref name="EdwardsJC20151101" /> | ||
In early 2016, [[Leonid Schneider]], a science journalist, criticised the lack of data sharing by the PACE trial authors and the ''Lancet'' and its editor Richard Horton's role in the scandal and compared it to other recent ''Lancet'' scandals.<ref name="Schneider20160405" /> <ref name="Schneider20160219" /> <ref name="SchneiderIndex" /> | In early 2016, [[Leonid Schneider]], a science journalist, criticised the lack of data sharing by the PACE trial authors and the ''Lancet'' and its editor Richard Horton's role in the scandal and compared it to other recent ''Lancet'' scandals.<ref name="Schneider20160405" /><ref name="Schneider20160219" /><ref name="SchneiderIndex" /> | ||
Prof [[Andrew Gelman]], Professor of Statistics and Political Science at | Prof [[Andrew Gelman]], Professor of Statistics and Political Science at Columbia University, NY, examined the Lancet's role in how the PACE trial got taken so seriously and stayed afloat for so long.<ref name="GelmanA20151223" /><ref name="GelmanA20151225" /><ref name="GelmanA20160105" /><ref name="GelmanA20170709" /> | ||
Dr [[Sten Helmfrid]] published an article in the journal | Dr [[Sten Helmfrid]] published an article in the journal ''Socialmedicinsk tidskrift'' in September 2016 called "Studies on Cognitive Behavioral Therapy and Graded Exercise Therapy for ME/CFS are misleading" that criticized not only the PACE trial, but the entire CBT/GET paradigm for ME/CFS. "The underlying model has no theoretical foundation and is at odds with physiological findings. Surveys suggest that the efficacy of CBT is no better than placebo and that GET is harmful. Therefore, cognitive behavioral therapy and graded exercise therapy for ME/CFS are not evidence based."<ref name="HelmfridS201609" /> | ||
Dr [[Sonia Lee]], a clinical epidemiologist, published 30 slides critically appraising the PACE trial in February 2017 and concluding the PACE trial authors should "rectify or retract".<ref name="LeeSonia-slide" /><ref name="LeeSonia-video" /> Dr Lee | Dr [[Sonia Lee]], a clinical epidemiologist, published 30 slides critically appraising the PACE trial in February 2017 and concluding the PACE trial authors should "rectify or retract".<ref name="LeeSonia-slide" /><ref name="LeeSonia-video" /> Dr Lee self-published ''Research Waste In ME/CFS'' which compared [[biopsychosocial model|psychosocial]] trials using the PACE trial as an example psychosocial trial. Lee found that psychosocial trials were more likely to engage in selective reporting than biomedical trials, that PACE in particular had significant design, evidence and justification weaknesses or omissions, and concluded "it confirms the concerns raised by ME/CFS groups that psychosocial interventions are [[medical eglect and abuse|harmful]], and present questionable therapeutic benefits no different to a placebo".<ref>{{cite journal | title = Research Waste in ME/CFS | first = Sonia | last = Lee | doi = 10.1101/133926 | url = https://www.biorxiv.org/content/early/2017/05/07/133926 | journal = biorxiv | date = May 7, 2017}}</ref> | ||
Professor [[Leonard Jason]] in the Journal of Health Psychology wrote in February 2017 | Professor [[Leonard Jason]] in the Journal of Health Psychology wrote in February 2017 'The PACE trial missteps on pacing and patient selection' which criticised the issue of patient selection in the trial with the use of the [[Oxford criteria]] included those without the disease and the PACE trial investigators did not design and implement a valid pacing intervention in the trial.<ref name="JasonLA201702" /> | ||
Dr [[Sarah Myhill]] in her August 2017 video on [[Sarah_Myhill#maimes |Medical Abuse in ME Sufferers (MAIMES)]] called the PACE trial "fraudulent" and "a criminal act" and is an "abuse" of "vulnerable people" and "defrauds them of benefits".<ref name="MyhillS201708-video" /> | Dr [[Sarah Myhill]] in her August 2017 video on [[Sarah_Myhill#maimes | Medical Abuse in ME Sufferers (MAIMES)]] called the PACE trial "fraudulent" and "a criminal act" and is an "abuse" of "vulnerable people" and "defrauds them of benefits".<ref name="MyhillS201708-video" /> | ||
In April 2018 Dr Neil McGregor | In April 2018, ME/CFS researcher Dr [[Neil McGregor]] sent his analysis of the harms in the PACE studies, to [[Australia]]'s Chief Medical Officer. In it, McGregor stated that "The conclusions within the PACE studies is consistent with a highly biased study which under-estimates, and does not assess, the adverse outcomes in relationship to the disease process. The use of therapies of this type cannot be recommended based upon the data within the PACE study and must exclude the worst affected patients as they were not included in the study assessments."<ref>{{cite web | url = https://www.facebook.com/MEAdvocacyNetworkAustralia/photos/a.1136337343101296/1350233075045054/?type=3 | title = Prominent Australian ME/CFS researcher, Neil McGregor, has weighed in on the GET debate | date = June 6, 2017 | publisher = ME Advocacy Network Australia | website = Facebook }}</ref><ref name="Harm-PACE-trial">{{Cite web | last = McGregor | first = Neil | authorlink = Neil McGregor | title = Harm and the PACE trial | website = Dropbox | url = https://www.dropbox.com/s/lrrz4gx5nnmo687/PACEStudyHarmAssessmentNM.pdf | date = 2017 }}</ref> | ||
===Scientists' open letter to The Lancet and Psychological Medicine=== | ====Scientists' open letter to The Lancet and Psychological Medicine==== | ||
In November 2015, scientists [[Ronald Davis]], [[David Tuller]], [[Vincent Racaniello]], [[Jonathan Edwards]], [[Leonard Jason]], [[Bruce Levin]] and [[Arthur Reingold]] wrote an open letter to The Lancet citing "major flaws" in the original trial publication and asking for an independent re-analysis of the individual-level trial data.<ref name="openletrLANCET1" /> The journal failed to respond. | In November 2015, scientists [[Ronald Davis]], [[David Tuller]], [[Vincent Racaniello]], [[Jonathan Edwards]], [[Leonard Jason]], [[Bruce Levin]] and [[Arthur Reingold]] wrote an open letter to The Lancet citing "major flaws" in the original trial publication and asking for an independent re-analysis of the individual-level trial data.<ref name="openletrLANCET1" /> The journal failed to respond. | ||
| Line 265: | Line 257: | ||
Richard Horton, the editor of the Lancet, requested it to be submitted as an official letter but after 6 months of chasing he rejected it and refused to publish the letter.<ref name="MEACTION20160905" /><ref name="TWIV20160821" /> | Richard Horton, the editor of the Lancet, requested it to be submitted as an official letter but after 6 months of chasing he rejected it and refused to publish the letter.<ref name="MEACTION20160905" /><ref name="TWIV20160821" /> | ||
On 13 | On March 13, 2017 [[David Tuller]] and the original signatories to the open letter to the Lancet in 2016 and an additional 37 signatories signed an open letter to the Journal of Psychological Medicine. In total 102 signatories signed this open letter regarding the recovery paper of 2013 to the Journal of Psychological Medicine. | ||
These included scientists and medical professionals including [[Molly Brown]], [[Todd Davenport | Todd E. Davenport]], [[Simon Duffy]], [[Meredyth Evans]], [[Robert Garry | Robert F. Garry]], [[Keith Geraghty]], [[Ian Gibson]], [[Rebecca Goldin]], [[Ellen Goudsmit]], [[Maureen Hanson]], [[Malcolm Hooper]], [[Betsy Keller]], [[Andreas Kogelnik | Andreas M. Kogelnik]], [[Eliana Lacerda | Eliana M. Lacerda]], [[Vincent Lombardi | Vincent C. Lombardi]], [[Alex Lubet]], [[Steven Lubet]], [[Patrick McKnight | Patrick E. McKnight]], [[Jose Montoya | Jose G. Montoya]], [[Henrik Nielsen]], [[Elisa Oltra]], [[Nicole Porter]], [[Anders Rosén]], [[Peter Rowe | Peter C. Rowe]], [[William Satariano]], [[Ola Saugstad | Ola Didrik Saugstad]], [[Eleanor Stein]], [[Staci Stevens]], [[Julian Stewart]], [[Leonie Sugarman]], [[Mark VanNess]], [[Mark Vink]], [[Frans Visser]], [[Tony Ward]], [[John Whiting]], [[Carolyn Wilshire]] and [[Michael Zeineh]].<ref name="various201308psymed">{{cite journal | last1 = Murray | first1 = Robin | authorlink1 = Robin Murray | last2 = Agardy | first2 = Susanna | authorlink2 = Susanna Agardy | last3 = Carter | first3 = Samuel | authorlink3 = Sam Carter | last4 = Courtney | first4 = Robert | authorlink4 = Robert Courtney | last5 = Cox | first5 = Duncan | authorlink5 = Duncan Cox | last6 = Maryhew | first6 = Carly | authorlink6 = Carly Maryhew | last7 = Shepherd | first7 = Charles | authorlink7 = Charles Shepherd | last8 = White | first8 = Peter | authorlink8 = Peter White | quote = Editorial note<br>Unusually for Psychological Medicine, we publish below six letters concerning the paper by White et al (2013) on the PACE Trial. The UK Office of the Journal received 15 letters criticising aspects of this paper, but it seemed unlikely that all of these letters originated entirely independently since a number arrived on consecutive days and reiterated the same points. Nevertheless, in the spirit of scientific openness, we have published six of the letters which cover the main criticisms, and invited Professor White to reply to them. | title = Letter to the Editor: Comments on 'Recovery from chronic fatigue syndrome after treatments given in the PACE trial' | journal = Journal of Psychological Medicine | volume = 43 | issue = 8 | pages = 1787-1787 | doi = | |||
10.1017/S003329171300113X | date = Jul 22, 2013 | url = | |||
http://journals.cambridge.org/action/displayAbstract?fromPage=online&aid=8955878&fulltextType=LT&fileId=S003329171300113X }}</ref><ref>{{Cite web | url = http://www.meassociation.org.uk/2013/07/pace-trial-letters-and-reply-journal-of-psychological-medicine-august-2013/ | title = PACE trial: letters and reply {{!}} Journal of Psychological Medicine {{!}} August 2013 | website = [[ME Association]] | date = Aug 23, 2013 }}</ref> Twenty-seven ME charities from seventeen countries also co-signed the open letter including [[European ME Alliance]], [[25 Percent ME Group]] (UK group for severe ME patients), [[Emerge Australia]], [[Irish ME Trust]], [[ME Association]] (UK), and [[Solve ME/CFS Initiative | International Solve ME/CFS Initiative]] (US).<ref name="openletrEMEA">{{cite web | author1 = European ME Alliance (EMEA) | authorlink1 = European ME Alliance | author2 = ME-Vereniging (Belgium) | authorlink2 = ME-Vereniging | author3 = Foreningen for Myalgisk Encefalomyelitis (Denmark) | authorlink3 = Foreningen for Myalgisk Encefalomyelitis | author4 = ME Foreningen (Denmark) | authorlink4 = ME Foreningen | author5 = Suomen CFS-Yhdistys (Finland) | authorlink5 = Suomen CFS-Yhdistys | author6 = Fatigatio e.V. (Germany) | authorlink6 = Fatigatio e.V. | author7 = Het Alternatief (Netherlands) | authorlink7 = Het Alternatief | author8 = ME félag Íslands (Iceland) | authorlink8 = ME félag Íslands | author9 = Irish ME Trust (Ireland) | authorlink9 = Irish ME Trust | author10 = Associazione Malati di CFS (Itali) | authorlink10 = Associazione Malati di CFS | author11 = Norges ME-forening (Norway) | authorlink11 = Norges ME Forening | author12 = Liga SFC (Spain) | authorlink12 = Liga SFC | author13 = Riksföreningen för ME-patienter (RME) (Sweden) | authorlink13 = Riksföreningen för ME-patienter | author14 = Verein ME/CFS Schweiz (Switzerland) | authorlink14 = Verein ME/CFS Schweiz | author15 = Invest in ME Research (UK) | authorlink15 = Invest in ME Research | title = Open Letter to QMUL - Request for the release of PACE trial data | date = Mar 12, 2016 | url = http://www.euro-me.org/Documents/Documents-EU/EMEA%20Letter%20PACE%20TRIAL%20QMUL%202016.pdf }}</ref> | |||
On March 23, 2017, David Tuller reported that one of the editors of the Journal of Psychological Medicine, Sir Robin Murray, responded with "an unacceptable response." Tuller restated that "That the editors of Psychological Medicine do not grasp that it is impossible to be “disabled” and “recovered” simultaneously on an outcome measure is astonishing and deeply troubling." The open letter was reposted with signatures from an additional 17 clinicians, scientists or advocates and 23 more charities including [[Norman Booth | Norman E. Booth]], [[Joan Crawford]], [[Valerie Eliot Smith]], [[Susan Levine]], and [[Sarah Myhill]]. | |||
No response from the Lancet was received - | An additional 25 patient charities to sign included Associated New Zealand ME Society, [[Deutsche Gesellschaft für ME/CFS]] and [[Lost Voices Stiftung]] (Germany), [[ME Research UK]], ME/CFS (Australia) Ltd, [[The MEAction Network]], [[Millions Missing Canada]], National CFIDS Foundation, Inc. (US), and [[Open Medicine Foundation]] (US). No response from the Lancet was received - campaigning in 2018 continued. | ||
===Petitions and Protests=== | ====Petitions and Protests==== | ||
On October 28, 2015, [[MEAction]] launched a petition addressed to The Lancet, Psychological Medicine and the PACE trial authors, calling for an independent analysis of the data and the retraction of some of the PACE trial's "misleading claims" based on "absurd 'normal ranges' for fatigue and physical function". The petition was closed in February 2016, having gathered 11,897 signatures from people in sixty-four countries.<ref name="MEACTIONpacepetition" /><ref name="MEACTIONpacepetitiondelivered" /> | On October 28, 2015, [[The MEAction Network]] launched a petition addressed to The Lancet, Psychological Medicine and the PACE trial authors, calling for an independent analysis of the data and the retraction of some of the PACE trial's "misleading claims" based on "absurd 'normal ranges' for fatigue and physical function". The petition was closed in February 2016, having gathered 11,897 signatures from people in sixty-four countries.<ref name="MEACTIONpacepetition" /><ref name="MEACTIONpacepetitiondelivered" /> | ||
A US petition to the [[Agency for Healthcare Research and Quality]] (AHRQ) and the [[Centers for Disease Control]] was also launched in late 2015, asking government agencies to remove guidelines and recommendations based on PACE and other studies using the [[Oxford definition]] of ME/CFS.<ref name="MEAction20151116petition" /> | A US petition to the [[Agency for Healthcare Research and Quality]] (AHRQ) and the [[Centers for Disease Control]] was also launched in late 2015, asking government agencies to remove guidelines and recommendations based on PACE and other studies using the [[Oxford definition]] of ME/CFS.<ref name="MEAction20151116petition" /> | ||
On [[International ME Awareness Day]] (May 12th) in 2017 due to the intransigence of the PACE researchers for years, a [[Millions Missing protests | Millions Missing protest]] in London resulted in hundreds of patients protesting against the PACE trial.<ref>{{Cite web | first = Catherine | last = Hale | authorlink = Catherine Hale | url = http://www.catherinehale.net/2017/05/millionsmissing-protest-london-120517.html?m=1 | title = #MillionsMissing protest London 12.05.17 | date = May 12, 2017 | access-date = 2022-10-06 }}</ref> | |||
===Breaches of patient's data security by study investigators=== | ====Breaches of patient's data security by study investigators==== | ||
In 2006 confidential PACE trial patient data was stolen from an unlocked drawer at King's College London.<ref name="pace20060331theft" /> | In 2006 confidential PACE trial patient data was stolen from an unlocked drawer at King's College London.<ref name="pace20060331theft" /> | ||
[[Malcolm Hooper]] documents in ''Magical Medicine'' how in 2005 confidential patient data was erroneously released by PACE author Professor [[Michael Sharpe]] in relation to another study.<ref name="HoopWill201002MM | [[Malcolm Hooper]] documents in ''Magical Medicine'' how in 2005 confidential patient data was erroneously released by PACE author Professor [[Michael Sharpe]] in relation to another study.<ref name="HoopWill201002MM" /> | ||
=== The Centre for Welfare Reform - 'In the Expectation of Recovery' === | ====The Centre for Welfare Reform - 'In the Expectation of Recovery'==== | ||
The [[Centre for Welfare Reform]] published a 64 page report in April 2016 examining the PACE trial and relating the study to the biopsychosocial model and its | The [[Centre for Welfare Reform]] published a 64 page report in April 2016 examining the PACE trial and relating the study to the biopsychosocial model and its links and influence from the insurance industry and government welfare reforms. The report titled [http://www.centreforwelfarereform.org/library/type/pdfs/in-the-expectation-of-recovery.html 'In the Expectation of Recovery'] by George Faulkner heavily criticised the PACE trial and stated "The way in which the biopsychosocial model has been used and promoted, without good supporting evidence for many of the claims being made, is unethical.” and “Had homeopaths or a pharmaceutical company conducted a trial and presented results in the manner of the PACE trial the British research community would have been unlikely to overlook its problems."<ref>{{Cite web | title = Centre for Welfare Reform criticises PACE trial | website = The MEAction Network | last = | first = | authorlink = The MEAction Network | access-date = October 6, 2022 | date = April 26, 2016 | url = http://www.meaction.net/2016/04/26/centre-for-welfare-reform-criticises-pace-trial }}</ref> Dr [[Simon Duffy]] writing in the Huffington Post questioned the motive of the research in 'The Misleading Research at the Heart of Disability Cuts'.<ref name="DuffyS20160420" /> | ||
=== Sense About Science USA === | ====Sense About Science USA==== | ||
Sense about Science USA (SAS USA) published a major statistical examination of the PACE trial in March 2016. The Executive Director of SAS USA, Trevor Butterworth wrote an accompanying editorial on PACE.<ref name="ButterworthT20160321" /> Prof Rebecca Goldin of Mathematical Sciences at George Mason University and Director of STATS (a collaboration between SAS USA and the American Statistical Association) wrote the 7000 word statistical critique PACE: The research that sparked a patient rebellion and challenged medicine.<ref name="GoldinR20160321" /> | |||
=== Other major investigations and reports | ====Other major investigations and reports==== | ||
In August 2016 [[Julie Rehmeyer]] presented a critique of the PACE trial at the largest gathering of statisticians in North America, the | In August 2016 [[Julie Rehmeyer]] presented a critique of the PACE trial at the largest gathering of statisticians in North America, the Joint Statistical Meeting 2016, in Chicago titled Bad Statistics, Bad Reporting, Bad Impact on Patients: The Story of the PACE Trial. Rehmeyer said "When I went through the slides showing the changes to the physical function criterion for recovery, I saw jaws drop."<ref name="Rehmeyer20160808" /> | ||
=== PACE Trial in UK Parliament === | ====PACE Trial in UK Parliament==== | ||
The PACE Trial has been the subject of a number of [http://www.parliament.uk/search/results/?pagesize=100&q=%22pace+trial%22&page=0 parliamentary enquiries] by parliamentarians mainly the [[Countess of Mar]]. The [[Countess of Mar]] asked questions of the government via a short debate in the House of Lords on the assessment of the PACE trial on 6 February 2013. A video and transcript is available on YouTube and Hansard.<ref name="UKParl20130206-video" /><ref name="UKParl20130206-transcript" /> Comments and analysis on the PACE trial and its establishment defenders has also been made by advocates.<ref name="HoopWill20130206" /> | The PACE Trial has been the subject of a number of [http://www.parliament.uk/search/results/?pagesize=100&q=%22pace+trial%22&page=0 parliamentary enquiries] by parliamentarians mainly the [[Countess of Mar]]. The [[Countess of Mar]] asked questions of the government via a short debate in the House of Lords on the assessment of the PACE trial on 6 February 2013. A video and transcript is available on YouTube and Hansard.<ref name="UKParl20130206-video" /><ref name="UKParl20130206-transcript" /> Comments and analysis on the PACE trial and its establishment defenders has also been made by advocates.<ref name="HoopWill20130206" /> | ||
During the court case | During the court case in 2015 by [[Alem Matthees | Matthees]] and the Information Commissioners Office v QMUL it was stated by [[Peter White]] that he regarded parliamentary debates as "harassment" and had to brief those taking part in the debate. | ||
In November 2016 [ | In November 2016, [[Kelvin Hopkins]] MP asked seven written questions relating to the PACE Trial including "request that the Medical Research Council conducts an inquiry into the management of the PACE trial to ascertain whether any fraudulent activity has occurred." and "prevent the PACE trial researchers from being given further public research funding until an inquiry into possible fraudulent activity into the PACE trial has been conducted."<ref name="Hopkins7">{{Cite web | last = Hopkins | first = Kelvin | authorlink = Kelvin Hopkins | title = Find written questions and answers | website = UK Parliament - Written questions, answers and statements | date = 2016 | access-date = 2010-10-06 | url = https://questions-statements.parliament.uk/written-questions?SearchTerm=Chronic+Fatigue+Syndrome&DateFrom=01%2F01%2F2016&DateTo=01%2F01%2F2017&AnsweredFrom=01%2F01%2F2016&AnsweredTo=01%2F01%2F2017&House=Bicameral&MemberId=2&Answered=Any&Expanded=True }}</ref> | ||
The Countess of Mar has also asked in February 2017 the Committee of Public Accounts to investigate the use of public funds on the PACE Trial and the PACE authors use of public funds to resist requests to release anonymised clinical trial data. Her letter included a report which stated the trial was "professional misconduct and/or fraud" and "Professor White obtained ethical approval for the study under false pretences".<ref name="HoopWill20170201" /> | The [[Countess of Mar]] has also asked in February 2017 the UK parliament's Committee of Public Accounts to investigate the use of public funds on the PACE Trial and the PACE authors use of public funds to resist requests to release anonymised clinical trial data. Her letter included a report which stated the trial was "professional misconduct and/or fraud" and "Professor White obtained ethical approval for the study under false pretences".<ref name="HoopWill20170201" /> | ||
==Response to criticism== | ===Response to criticism=== | ||
===Response=== | ====Response==== | ||
The trial investigators have replied to some criticism of the trial but have been much criticised as being evasive and not responding to the issues raised. | The trial investigators have replied to some criticism of the trial but have been much criticised as being evasive and not responding to the issues raised. | ||
Some of their | Some of their response to letters to The Lancet concerning their main analyses,<ref name="pace20110517corr" /> Psychological Medicine concerning their recovery analyses,<ref name="various201308psymed" /> and Lancet Psychiatry concerning their secondary mediation analyses <ref name="pace2015corrlanpsy0114-5" /> and long-term follow-up paper.<ref name="pace2016corrlanpsy00018-3" /> A letter to the BMJ by Tom Kindlon<ref name="Kindlon20130830bmj6559900" /> drew a reply from the authors<ref name="pace2013corrbmj664559" /> that in turn received 31 responses of its own.<ref name="various2013bmjf5963" /> | ||
Since the controversy with criticism from the scientific community they have largely not responded to the key concerns and have repeated their earlier arguments. | Since the controversy with criticism from the scientific community they have largely not responded to the key concerns and have repeated their earlier arguments. | ||
| Line 328: | Line 316: | ||
Professor [[James Coyne]] reported that he agreed to debate the authors on health website National Elf about the trial but that they declined.<ref name="Coyne20151104" /> Professor [[Simon Wessely]] was given the vacated National Elf spot and wrote a lengthy article praising the trial, noting that he once described it as "a thing of beauty" and saying, "We can accept that PACE was a good trial and we can have some confidence in its findings".<ref name="paceWesselyTitanic" /> | Professor [[James Coyne]] reported that he agreed to debate the authors on health website National Elf about the trial but that they declined.<ref name="Coyne20151104" /> Professor [[Simon Wessely]] was given the vacated National Elf spot and wrote a lengthy article praising the trial, noting that he once described it as "a thing of beauty" and saying, "We can accept that PACE was a good trial and we can have some confidence in its findings".<ref name="paceWesselyTitanic" /> | ||
===Allegations of harassment, death threats and smear campaign=== | ====Allegations of harassment, death threats and smear campaign==== | ||
During the criticism of the PACE trial by patient groups the PACE trial psychiatrists publicised that they were receiving death threats and harassment. | During the criticism of the PACE trial by patient groups the PACE trial psychiatrists publicised that they were receiving death threats and harassment. | ||
The PACE trial investigators and Professor [[Simon Wessely]] have publicly claimed they have been harassed and subjected to death threats.<ref name="Guardian20110821" /><ref name="paceWessely20110827" /><ref name="paceWessely20110729" /><ref name="ZimmerC20110821" /><ref name="ScienceMag20110906" /><ref name="Telegraph20110729" /><ref name="StuffNon20121117" /> <ref name="BBCUK20110721" /> A feature article in the BMJ (read by most UK doctors) was published in June 2011 called 'Dangers of research into chronic fatigue syndrome'.<ref name="Hawkes2011" /> The Times article was titled | The PACE trial investigators and Professor [[Simon Wessely]] have publicly claimed they have been harassed and subjected to death threats.<ref name="Guardian20110821" /><ref name="paceWessely20110827">{{cite news | last1 = Wessely | first1 = Simon | authorlink1 = Simon Wessely | title = Mind the gap - It's time to stop separating psychiatry and neurology | publisher = Spectator (UK) | date = Aug 27, 2011 | url = http://www.spectator.co.uk/2011/08/mind-the-gap-3/}}</ref><ref name="paceWessely20110729" /><ref name="ZimmerC20110821" /><ref name="ScienceMag20110906" /><ref name="Telegraph20110729" /><ref name="StuffNon20121117" /><ref name="BBCUK20110721" /> A feature article in the BMJ (read by most UK doctors) was published in June 2011 called 'Dangers of research into chronic fatigue syndrome'.<ref name="Hawkes2011" /> ''The Times'' article was titled 'Doctor’s hate mail is sent by the people he tried to cure'.<ref name="paceWessely20110806" /> An article was also published in the ''Sunday Times'' magazine with Simon Wessely repeating the death threats narrative.<ref name="SunTimesUK20130505" /> One article was even called ‘It’s safer to insult the Prophet Mohammed than to contradict the armed wing of the ME brigade’.<ref name="Telegraph20120928" /> | ||
However, the PACE authors and their supporters have been accused of blurring the line between harassment and legitimate criticism of the study. Documentation obtained under the Freedom of Information Act from meetings in 2013 that were attended by some of PACE’s principal investigators include a statement that “harassment is most damaging in the form of vexatious FOIs [Freedom of Information requests].”<ref name="TymesTrust2014bts" /> This framing of FOIA requests as harassment is widely taken to be a reference to the PACE authors, who have complained about the number of FOI requests that they have received for data<ref name="FOI20150318" /> and who have dismissed several as "vexatious":<ref name="FOI20151211JC" /><ref name="FOI20151101AS" /><ref name="FOI20150629GM-c" /> the Information Commissioner's Office was told that Professor Peter White | However, the PACE authors and their supporters have been accused of blurring the line between harassment and legitimate criticism of the study. Documentation obtained under the Freedom of Information Act from meetings in 2013 that were attended by some of PACE’s principal investigators include a statement that “harassment is most damaging in the form of vexatious FOIs [Freedom of Information requests].”<ref name="TymesTrust2014bts" /> This framing of FOIA requests as harassment is widely taken to be a reference to the PACE authors, who have complained about the number of FOI requests that they have received for data<ref name="FOI20150318" /> and who have dismissed several as "vexatious":<ref name="FOI20151211JC" /><ref name="FOI20151101AS" /><ref name="FOI20150629GM-c" /> the Information Commissioner's Office was told that Professor Peter White "believes that the requests are clearly part of a campaign to discredit the trial” and that “the effect of these requests has been that the team involved in the PACE trial, and in particular the professor involved, now feel harassed and believe that the requests are vexatious in nature."<ref name="FOI20150318" /> | ||
The criticisms of the trial's methodology and analyses by patients and others has been referred to by the investigators - and The Lancet - as part of a campaign to undermine the the study. In an editorial comment that accompanied letters criticising the trial, The Lancet described the trial as “rigorously conducted” and questioned whether the “coordination of the response... has been born... from an active campaign to discredit the research”.<ref name="pace2011LancetEd" /> In an interview on Australian national radio shortly after publication, Dr Richard Horton, The Lancet’s editor, described patients who criticised the trial as “a fairly small, but highly organised, very vocal and very damaging group of individuals”.<ref name="pace20110418radio" / | The criticisms of the trial's methodology and analyses by patients and others has been referred to by the investigators - and The Lancet - as part of a campaign to undermine the the study. In an editorial comment that accompanied letters criticising the trial, The Lancet described the trial as “rigorously conducted” and questioned whether the “coordination of the response... has been born... from an active campaign to discredit the research”.<ref name="pace2011LancetEd">{{citation | last1 = The Lancet | title = Editorial: Patients' power and PACE | journal = The Lancet | date = May 17, 2011 | doi = 10.1016/S0140-6736(11)60696-X | url = http://www.thelancet.com/journals/lancet/article/PIIS0140-6736(11)60696-X/fulltext}}</ref> In an interview on Australian national radio shortly after publication, Dr Richard Horton, The Lancet’s editor, described patients who criticised the trial as “a fairly small, but highly organised, very vocal and very damaging group of individuals”.<ref name="pace20110418radio">{{citation | last1 = Swan | first1 = Norman | authorlink1 = Norman Swan | last2 = Sharpe | first2 = Michael | authorlink2 = Michael Sharpe | last3 = Horton | first3 = Richard | authorlink3 = Richard Horton | title = Health Report - Comparison of treatments for chronic fatigue syndrome - the PACE trial | journal = ABC Radio National (Australia) - Health Report | date = Apr 18, 2011 | url = http://www.abc.net.au/radionational/programs/healthreport/comparison-of-treatments-for-chronic-fatigue/2993296#transcript | url-status = dead | archive-date = Apr 18, 2011 | archive-url = https://web.archive.org/web/20110423065733/http://mpegmedia.abc.net.au/rn/podcast/2011/04/hrt_20110418_0830.mp3}}</ref> | ||
But some accuse the investigators of a campaign against patients, labelling them as harassers to undermine their criticisms of the trial, including [[Angela Kennedy]].<ref name="KennedyAng20121119" /> | But some accuse the investigators of a campaign against patients, labelling them as harassers to undermine their criticisms of the trial, including [[Angela Kennedy]].<ref name="KennedyAng20121119" /> | ||
The PACE trial authors refused to provide anonymised data to many individuals and also refused to accept the Information Commissioners Office decision for QMUL to release the data in 2015 (see Release of Data/Information Tribunal below). During the PACE trial authors appeal to the Tribunal an article | The PACE trial authors refused to provide anonymised data to many individuals and also refused to accept the Information Commissioners Office decision for QMUL to release the data in 2015 (see Release of Data/Information Tribunal below). During the PACE trial authors appeal to the Tribunal an article was published during this period by their associates in ''Nature'' in which they bizarrely appeared to use projection in describing disabled ME sufferers as “hard-line opponents” of research into chronic fatigue syndrome and compared them with industry lobbyists such as tobacco and climate change denialists.<ref name="Lewandowsky20160125" /><ref name="Lewandowsky20160204" /> Public health expert and journalist [[David Tuller]], who has said, "Wrapping themselves in victimhood, the [PACE authors] have even managed to extend their definition of harassment to include any questioning of their science and the filing of requests for data — a tactic that has shielded their work from legitimate and much-needed scrutiny."<ref name="Tuller20160201" /><ref name="MEAction20160201victim" /> | ||
[https:// | The [[Science Media Centre]] (SMC) was found to have orchestrated and publicised the false narrative in 2011 in the UK media about extremists harassing researchers.<ref name="SMCUK201110TOP" /><ref name="SMCUK201302ETE" /> An article in the Establishment in May 2016, [https://theestablishment.co/the-hidden-battle-for-the-rights-of-chronic-fatigue-syndrome-sufferers-cd20bef0f13a#.nz06llmkn The Hidden Battle For The Rights Of Chronic Fatigue Syndrome Sufferers] summarised the campaign to smear ME sufferers and how these psychiatrists after categorising ME as a psychological over two decades then were able to use institutional gaslighting when patients were question the scientific validity of the trial and to stop access to data requests from the PACE trial by framing them as harassment and abuse. [[Catherine Hale]] has written about the 'Politics of Stigma' created for ME sufferers by the PACE trial authors.<ref name="HaleC201512" /> | ||
Peter Tatchell, a human rights advocate has supported ME sufferers for their human rights against the psychotherapies and the PACE trial and defended them from the smear campaigns by the psychiatrists similar to what he faced in his advocacy in the 1970s.<ref name="MEMilitant20171008" /><ref name="TatchellP20160921" /><ref name="TatchellP20160923" /> Ethics experts [[Charlotte Blease]] and [[Diane O'Leary]] have investigated ethics and injustice in the behavior of some researchers in the ME/CFS field and the effects of government and institutional actions on patients. | |||
No evidence of anyone with ME/CFS in relation to these matters being charged by the police/law enforcement or convicted in the Courts with harassment and death threats has come to light, despite a number of [[ME activists and advocates|ME advocates]] attempting to find evidence, including filing Freedom of Information Act requests to public bodies.<ref name="warringfactions">{{Cite web|url=https://valerieeliotsmith.com/2019/01/14/changing-the-narrative-2-warring-factions-divide-rule-and-death-threats/|title=Changing the narrative #2: warring factions, divide & rule and death threats|date=2019-01-14|website= | No evidence of anyone with ME/CFS in relation to these matters being charged by the police/law enforcement or convicted in the Courts with harassment and death threats has come to light, despite a number of [[ME activists and advocates | ME advocates]] attempting to find evidence, including filing Freedom of Information Act requests to public bodies.<ref name="warringfactions">{{Cite web | first = Valerie | last = Eliot Smith | authorlink = Valerie Eliot Smith | url = https://valerieeliotsmith.com/2019/01/14/changing-the-narrative-2-warring-factions-divide-rule-and-death-threats/ | title = Changing the narrative #2: warring factions, divide & rule and death threats | date = 2019-01-14 | website = ValerieEliotSmith | language = en | access-date = 2019-08-14}}</ref><ref name="warringfactions" /><ref name="FOIA138299">{{Cite web | url = https://www.whatdotheyknow.com/request/138299/response/341512/attach/html/2/Response%20Letter.pdf.html | title = Freedom of information act request 138299, response 341512 | last = King's College London | first = | authorlink = King's College London | date = Dec 12, 2012 | website = Whatdotheyknow | archive-url = | archive-date = | url-status = | access-date = 2019-03-22}}</ref><ref name="FOIA138299" /><ref name="FOI370916">{{Cite web | url = https://www.whatdotheyknow.com/request/370916/response/921879/attach/3/F366.16%20Response.pdf | title = Freedom of Information Act request 370916, response 921879 | last = King's College London | first = | authorlink = King's College London | date = Jan 17, 2017 | website = | archive-url = | archive-date = | url-status = | access-date = }}</ref> | ||
{{See also | Intimidation and bullying of PACE trial critics}} | |||
==Calls to release data== | ===Calls to release data=== | ||
===Open data commitments=== | ====Open data commitments==== | ||
The study authors have been criticised for failing to follow the requirements of the [[Medical Research Council]] (who provided significant funding for the trial) to release anonymised trial data.<ref name="Schneider20160219comm" /> | The study authors have been criticised for failing to follow the requirements of the [[Medical Research Council]] (who provided significant funding for the trial) to release anonymised trial data.<ref name="Schneider20160219comm" /> | ||
The 2012 PACE cost-effectiveness paper<ref name="pace2012CE" /> was published in the journal [[PLoS One]]. That journal requires authors, as a condition of submitting their papers, to agree to release the anonymized trial data underlying the paper's analyses upon request. In November 2015 Professor [[James Coyne]] made a request to the PACE authors on that basis, but the authors treated his request as a Freedom of Information request and refused it.<ref name="Coyne20151204" /><ref name="Coyne20160214" /> However in March 2016 the [[PLoS One]] journal confirmed they had requested the investigators to release the trial data, as they committed to do prior to publication.<ref>{{Cite journal|last=McCrone|first=Paul|last2=Sharpe|first2=Michael|last3=Chalder|first3=Trudie|last4=Knapp|first4=Martin|last5=Johnson|first5=Anthony L.|last6=Goldsmith|first6=Kimberley A.|last7=White|first7=Peter D.|date=2012-08-01|editor-last=van Baal|editor-first=Pieter H. M.|title=Adaptive Pacing, Cognitive Behaviour Therapy, Graded Exercise, and Specialist Medical Care for Chronic Fatigue Syndrome: A Cost-Effectiveness Analysis|url=https://dx.plos.org/10.1371/journal.pone.0040808|journal=PLoS ONE|language=en|volume=7|issue=8|pages=e40808|doi=10.1371/journal.pone.0040808|issn=1932-6203|pmc= | The 2012 PACE cost-effectiveness paper<ref name="pace2012CE" /> was published in the journal [[PLoS One]]. That journal requires authors, as a condition of submitting their papers, to agree to release the anonymized trial data underlying the paper's analyses upon request. In November 2015 Professor [[James Coyne]] made a request to the PACE authors on that basis, but the authors treated his request as a Freedom of Information request and refused it.<ref name="Coyne20151204" /><ref name="Coyne20160214" /> However in March 2016 the [[PLoS One]] journal confirmed they had requested the investigators to release the trial data, as they committed to do prior to publication.<ref>{{Cite journal | last = McCrone | first = Paul | last2 = Sharpe | first2 = Michael | last3 = Chalder | first3 = Trudie | last4 = Knapp | first4 = Martin | last5 = Johnson | first5 = Anthony L. | last6 = Goldsmith | first6 = Kimberley A. | last7 = White | first7 = Peter D. | date = 2012-08-01 | editor-last = van Baal | editor-first = Pieter H.M. | title = Adaptive Pacing, Cognitive Behaviour Therapy, Graded Exercise, and Specialist Medical Care for Chronic Fatigue Syndrome: A Cost-Effectiveness Analysis | url = https://dx.plos.org/10.1371/journal.pone.0040808 | journal = PLoS ONE | language = en | volume = 7 | issue = 8 | pages = e40808 | doi = 10.1371/journal.pone.0040808 | issn = 1932-6203 | pmc = 3411573 | pmid = 22870204}}</ref><ref name="Coyne20160307" /> | ||
In 2015 Peter White lobbied the UK government through his university QMUL and the [[Science Media Centre]] | In 2015 Peter White lobbied the UK government through his university QMUL and the [[Science Media Centre]] to restrict the Freedom of Information Act 2000 for universities especially for controversial research and cited the PACE trial and compared ME patients requesting data with "climate change science, and research into the health effects of tobacco".<ref name="SMCUK20131031" /><ref name="PhoenixRisingPWFOI" /> | ||
===Freedom of Information requests=== | ====Freedom of Information requests==== | ||
The PACE trial investigators have been asked for 160 pieces of separate information in 35 Freedom of Information requests since 2011.<ref name="FOI20150318" /> They have dismissed at least three of these claims as "vexatious".<ref name="FOI20151211JC" /><ref name="FOI20151101AS" /><ref name="FOI20150629GM-c" /><ref name="FOI20160309AS" /><ref name="FOI20160329" /> | The PACE trial investigators have been asked for 160 pieces of separate information in 35 Freedom of Information requests since 2011.<ref name="FOI20150318" /> They have dismissed at least three of these claims as "vexatious".<ref name="FOI20151211JC" /><ref name="FOI20151101AS" /><ref name="FOI20150629GM-c" /><ref name="FOI20160309AS" /><ref name="FOI20160329" /> | ||
A ruling by a UK government body, the | A ruling by a UK government body, the Information Commissioner's Office (ICO), on October 27, 2015 ordered [[Queen Mary University of London]] (QMUL, the institutional base of the PACE trial's lead investigator [[Peter White]]) to release the trial data to a patient who had requested it, subject to appeal within 28 days.<ref name="FOI20151027QMUL" /> Three Freedom of Information requests from 2012 and 2013 were included in the ICO's decision.<ref name="FOI20121026" /><ref name="FOI20130903" /><ref name="FOI20131029C" /> An appeal by QMUL was heard by the First-Tier Tribunal on 20-22 April 2016. QMUL responded to a freedom of information request confirming the cost of its legal fees for the tribunal totalled £245,745.27 (around USD 350,000).<ref name="johnthejack20160629" /><ref name="MEACTION20160709qmul" /><ref name="EliotSmith20160715" /> The First-Tier Tribunal judgement was published on August 16, 2016, roundly dismissing the appeal by QMUL, and deciding that the PACE trial data should be released.<ref name="FOI20160812" /><ref name="EliotSmith20160816" /> The university has not yet stated whether it will appeal the judgement.<ref name="QMUL20160816" /> The PACE patient consent form was also the released through a Freedom of Information request.<ref name="FOI20140324M-b" /> | ||
The | Dr [[Richard Horton]], editor of [[The Lancet]], stated on April 18, 2011 in a national Australian radio interview: "The Freedom of Information requests and the legal fees that have been racked up over the years because of these vexatious claims has added another £750,000 of taxpayers’ money to the conduct of this study".<ref name="HoopWill20110528" /> | ||
====Data requests from scientists==== | |||
===Data requests from scientists=== | |||
Professor [[James Coyne]] has publicly called for the trial data to be released. He has repeatedly criticized the PACE investigators for failing to abide by modern expectations concerning "open data".<ref name="Coyne20151111" /><ref name="Coyne20151202" /><ref name="Coyne20151218" /><ref name="Coyne20151222" /><ref name="Coyne20160102" /><ref name="Coyne20160131" /><ref name="Coyne20160220" /><ref name="Coyne20160309" /><ref name="Coyne20160326" /> | Professor [[James Coyne]] has publicly called for the trial data to be released. He has repeatedly criticized the PACE investigators for failing to abide by modern expectations concerning "open data".<ref name="Coyne20151111" /><ref name="Coyne20151202" /><ref name="Coyne20151218" /><ref name="Coyne20151222" /><ref name="Coyne20160102" /><ref name="Coyne20160131" /><ref name="Coyne20160220" /><ref name="Coyne20160309" /><ref name="Coyne20160326" /> | ||
| Line 374: | Line 360: | ||
Professor Coyne's own request for the data was dismissed under the Freedom of Information Act by the study authors as "vexatious" and as having an "improper motive".<ref name="FOI20151211JC" /> | Professor Coyne's own request for the data was dismissed under the Freedom of Information Act by the study authors as "vexatious" and as having an "improper motive".<ref name="FOI20151211JC" /> | ||
Scientists [[Ronald Davis]], [[David Tuller]], [[Bruce Levin]], and [[Vincent Racaniello]] requested the PACE trial data in December 2015.<ref name="openletrQMUL1" /> Their request was rejected by the trial investigators.<ref name="Tuller201601119v" /> | Scientists [[Ronald Davis]], [[David Tuller]], [[Bruce Levin]], and [[Vincent Racaniello]] requested the PACE trial data in December 2015.<ref name="openletrQMUL1" /> Their request was rejected by the trial investigators.<ref name="Tuller201601119v" /> On International ME Awareness day in May 2016 in an interview with an advocate's article called [http://www.occupycfs.com/2016/05/12/pace-gate/ PACE-Gate], Dr Racaniello stated "I think they are going to ignore, obfuscate, and give their usual responses until we are all dead. I don’t have hope that the PACE authors, or Lancet, will respond in any meaningful way until there is more of an outcry."<ref name="MEAction20160514" /> | ||
===ME Charities and others calls for data release=== | ====ME Charities and others calls for data release==== | ||
A number of patient charity groups and individuals have called for the release of the PACE data, including some outside the ME/CFS community who advocate "open data" in science. These calls include: | A number of patient charity groups and individuals have called for the release of the PACE data, including some outside the ME/CFS community who advocate "open data" in science. These calls include: | ||
*2016: open letters from two patient charities, | *2016: open letters from two patient charities, in Australia, ME/CFS Australia (SA) Inc and Emerge Australia, calling for the data to be released.<ref name="openletrEmergeAust" /><ref name="openletrMECFSSthAust" /> | ||
*2016: an open letter the European ME Alliance (EMEA) representing patient group charities from 12 European countries (Belgium, Denmark, Finland, Germany, Holland, Iceland, Ireland, Norway, Spain, Sweden, Switzerland and UK), calling for the data to be released.<ref name="openletrEMEA" /> | *2016: an open letter the European ME Alliance (EMEA) representing patient group charities from 12 European countries (Belgium, Denmark, Finland, Germany, Holland, Iceland, Ireland, Norway, Spain, Sweden, Switzerland and UK), calling for the data to be released.<ref name="openletrEMEA" /> | ||
*2016: an open letter by [[Mark Berry]] of [[Phoenix Rising]], a US based non profit with the largest ME patient forum membership in the world, calling for the data to be released.<ref name="openletrPHOENIX" /> | *2016: an open letter by [[Mark Berry]] of [[Phoenix Rising]], a US based non profit with the largest ME patient forum membership in the world, calling for the data to be released.<ref name="openletrPHOENIX" /> | ||
| Line 386: | Line 372: | ||
*2016: an open letter from the Irish ME Trust charity.<ref name="openletrIMET" /> | *2016: an open letter from the Irish ME Trust charity.<ref name="openletrIMET" /> | ||
*2016: an open letter from a Canadian patient group, the [[National ME/FM Action Network]].<ref name="openletrMEFMANCAN" /> | *2016: an open letter from a Canadian patient group, the [[National ME/FM Action Network]].<ref name="openletrMEFMANCAN" /> | ||
*2016: an open letter from two Belgian patient organisations, | *2016: an open letter from two Belgian patient organisations, ME-Gids and WUCB.<ref name="openletrBelg" /> | ||
*2016: an open letter from a Dutch patient group, Groep ME-DenHaag / The Dutch Citizen’s Initiative for ME patients<ref name="openletrNeth1" /> and a joint letter from three further dutch patient group charities, ME/CVS Vereniging, ME/CVS Stichting Nederland, Steungroep ME en Arbeidsongeschiktheid<ref name="openletrNeth2" /> | *2016: an open letter from a Dutch patient group, Groep ME-DenHaag / The Dutch Citizen’s Initiative for ME patients<ref name="openletrNeth1" /> and a joint letter from three further dutch patient group charities, ME/CVS Vereniging, ME/CVS Stichting Nederland, Steungroep ME en Arbeidsongeschiktheid<ref name="openletrNeth2" /> | ||
*2016: an article, "[https://forbetterscience.wordpress.com/2016/02/08/pace-trial-and-other-clinical-data-sharing-patient-privacy-concerns-and-parasite-paranoia/ PACE trial and other clinical data sharing: patient privacy concerns and parasite paranoia]", by [[Leonid Schneider]], an independent science journalist.<ref name="Schneider20160219" /> | *2016: an article, "[https://forbetterscience.wordpress.com/2016/02/08/pace-trial-and-other-clinical-data-sharing-patient-privacy-concerns-and-parasite-paranoia/ PACE trial and other clinical data sharing: patient privacy concerns and parasite paranoia]", by [[Leonid Schneider]], an independent science journalist.<ref name="Schneider20160219" /> | ||
*2015: a blog post by Dr [[Richard Smith]], former editor of [[The BMJ]], who said that the PACE authors' institutions were "making a mistake .. the inevitable conclusion is that they have something to hide."<ref name="SmithR20151216" /> and in article for F1000 Research.<ref name="SmithR20160429" /> | *2015: a blog post by Dr [[Richard Smith]], former editor of [[The BMJ]], who said that the PACE authors' institutions were "making a mistake .. the inevitable conclusion is that they have something to hide."<ref name="SmithR20151216" /> and in article for F1000 Research.<ref name="SmithR20160429" /> | ||
*2016, [https://jcoynester.wordpress.com/2016/08/18/release-the-pace-trial-data-my-submission-to-the-uk-tribunal/ Release the PACE trial data: My submission to the UK Tribunal], James Coyne, 18 | *2016, [https://jcoynester.wordpress.com/2016/08/18/release-the-pace-trial-data-my-submission-to-the-uk-tribunal/ Release the PACE trial data: My submission to the UK Tribunal], James Coyne, Aug 18, 2016.<ref name="Coyne20160818" /> | ||
A total of 29 patient charity groups from 15 countries wrote in support of releasing the anonymized PACE trial data.<ref name="EllisC20160125" /><ref name="EllisC20160829" /> | A total of 29 patient charity groups from 15 countries wrote in support of releasing the anonymized PACE trial data.<ref name="EllisC20160125" /><ref name="EllisC20160829" /> | ||
A ME charity polled on the question "Should or should not the anonymised data from the PACE trial be released for independent analysis?" | A ME charity polled on the question "Should or should not the anonymised data from the PACE trial be released for independent analysis?" By March 17, 2016, 1391 voters took part and 99% (1378 voters) voted for 'Should be released'. 0% (5 voters) voted for 'Should not be released'.<ref name="MEASSUK201603poll" /> | ||
Both cofounders of Retraction Watch, [https://journalism.nyu.edu/about-us/profile/ivan-oransky-md/ Ivan Oransky] and Adam Marcus, added their weight behind patients in the refusal of the PACE trial investigators to release the anonymised data in article in STAT News feature 'The Watchdogs Keeping an eye on misconduct, fraud, and scientific integrity' [https://www.statnews.com/2015/12/23/sharing-data-science/ To keep science honest, study data must be shared] and concluded "when researchers refuse to share data, and how they came up with it, they lose the right to call what they do science".<ref name="GelmanA20151223" /> | |||
[[ | This battle was reported in the Wall Street Journal by Amy Dockser Marcus on 7 March 2016 as [http://www.wsj.com/articles/patients-scientists-fight-over-research-data-access-1457394712 Patients, Scientists Fight Over Research-Data Access], which featured quotes from [[David Tuller]], [[Tom Kindlon]], Anna Sheridan Wood, [[James Coyne]].<ref name="WSJ20160307">{{cite news | publisher = Wall Street Journal | first = Amy | last = Dockser Marcus | title = Patients, Scientists Fight Over Research-Data Access | date = Mar 7, 2016 | url = http://www.wsj.com/articles/patients-scientists-fight-over-research-data-access-1457394712 }}</ref> Professor Peter White responded to the article with a letter to the WSJ published 24th of March, 2016, claiming that "The main reason that my colleagues and I have been unable to release data to members of the public who ask for it is that we don’t have the consent of the trial participants to release their data in this way, and we are ethically bound to act in the best interest of our patients."<ref name="MEASSUK201603WSJ">{{cite web | last1 = ME Association | authorlink1 = ME Association | title = Patients, Scientists Fight Over Research-Data Access (Wall Street Journal) | date = Mar 7, 2016 | url = http://www.meassociation.org.uk/2016/03/patients-scientists-fight-over-research-data-access-wall-street-journal-7-march-2016/}}</ref> | ||
=== | ===Release of Data=== | ||
[[Alem Matthees]], an Australian ME sufferer, submitted a FOIA request to the PACE trial authors who were ordered to provide the requested data by the Information Commissioners Office. This decision was appealed by them to the Information Tribunal and was heard on April 20th 2016 at a three day hearing. | |||
====Information Tribunal==== | |||
[https:// | Mr [[Alem Matthees]]' original PACE trial data Freedom of Information Act request for the release of PACE trial data to Queen Mary University of London (QMUL) was submitted on March 24, 2014. QMUL refused to release the requested data and Mathees complained to the Information Commissioners Office. The Information Commissioners decision was made on October 27, 2015 and concluded that QMUL should release the data.<ref name="FirstTierTribunal2015">{{Cite news | title = First-tier tribunal: Information Rights Appeal EA/2015/0269 | url = https://informationrights.decisions.tribunals.gov.uk//DBFiles/Decision/i1854/Queen%20Mary%20University%20of%20London%20EA-2015-0269%20(12-8-16).PDF | page = 40 | last = Kennedy | first = Brian | last2 = Stephenson | first2 = Darryl | last3 = Watson | first3 = Nigel | quote = The evidence of <nowiki>[expert witness]</nowiki> Professor Anderson that third parties could not identify participants from the information alone and that, when pressed, he said that the chance of an "activist" being able to discover information that would lead to individual identification was remote, it was clear that his assessment of activist behaviour was, in our view, grossly exaggerated and the only actual evidence was that an individual at a seminar had heckled Professor Chalder. The identity of those questioning the research, who had signed an open letter or supported it, was impressive.}}</ref><ref name="FOI20151027QMUL">{{citation | last1 = Information Commissioner's Office, UK | title = FOI Decision Notice: to QMUL | date = Oct 27, 2015 | url = http://www.meaction.net/wp-content/uploads/2015/05/fs_50565190.pdf }}</ref> The PACE investigators appealed on November 23, 2015. | ||
QMUL appealed the decision, wishing to continue to withhold the PACE trial data, which led to a tribunal in 2016.<ref>{{cite web | last1 = Eliot Smith | first1 = Valerie | authorlink1 = Valerie Eliot Smith | website = ValerieEliotSmith Blog | url = https://valerieeliotsmith.com/2015/11/26/queen-mary-university-of-london-to-appeal-information-commissioners-decision-on-disclosure-of-pace-trial-data/ | date = Nov 26, 2015 | title = Queen Mary University of London to appeal Information Commissioner’s decision on disclosure of PACE Trial data }}</ref><ref name="noticeappeal">{{cite web | website = ValerieEliotSmith Blog | url = https://valerieeliotsmith.files.wordpress.com/2016/04/002-231115-notice-of-appeal.pdf | last = QMUL | title = Notice of Appeal | date = November 23, 2015}}</ref> The Information Commissioner's response to the Tribunal as First Respondent of January 12, 2016 and Mr Alem Mathees Main response to Tribunal as Second Respondent was also submitted to the Tribunal for the three day hearing from April 20th-22nd, 2016.<ref>{{cite web | last1 = Eliot Smith | first1 = Valerie | authorlink1 = Valerie Eliot Smith | website = ValerieEliotSmith Blog | url = https://valerieeliotsmith.files.wordpress.com/2016/04/010-090216-r2-matthees_main_response.pdf | date = April 2016 | title = Mr Alem Mathees Main response to Tribunal as Second Respondent }}</ref> The Information Rights Tribunal Judgement was finally published on August 16, 2016.<ref name="EliotSmith20160816">{{cite web | last1 = Eliot Smith | first1 = Valerie | authorlink1 = Valerie Eliot Smith | title = Tribunal orders release of PACE Trial data (QMUL v the IC and Matthees) | website = ValerieEliotSmith Blog | date = Aug 16, 2016 | url = https://valerieeliotsmith.com/2016/08/16/tribunal-orders-release-of-pace-trial-data-qmul-v-the-ic-and-matthees/ }}</ref><ref>{{Cite web | url = http://informationrights.decisions.tribunals.gov.uk/DBFiles/Decision/i1854/Queen%20Mary%20University%20of%20London%20EA-2015-0269%20(12-8-16).PDF | title = Appeal Number EA/2015/0269 Queen Mary University of London vs The Information Commissioner and Alem Matthees {{!}} First-Tier Tribunal {{!}} Information Rights | date = August 16, 2016 | publisher = Information Commissioner's Office | archive-date = 2016 | url-status = live | archive-url = https://dl.dropboxusercontent.com/u/23608059/OCR-Queen%20Mary%20University%20of%20London%20EA-2015-0269%20%2812-8-16%29.pdf }}</ref> The Tribunal upheld the original ICO decision and rejected the appeal by the PACE investigators and ordered QMUL to release the anonymised data to Mr Matthees. | |||
The tribunal took evidence under the normal rules of court. The tribunal also concluded of the expert witness for the PACE authors that "It was clear that his assessment of activist behaviour was, in our view, grossly exaggerated and the only actual evidence was that an individual at a seminar had heckled Professor | The tribunal took evidence under the normal rules of court. The tribunal also concluded of the expert witness for the PACE authors that "It was clear that his assessment of activist behaviour was, in our view, grossly exaggerated and the only actual evidence was that an individual at a seminar had heckled Professor Chalder" and "clearly in our view had some self-interest, exaggerated his evidence and did not seem to us to be entirely impartial. What we got from him was a considerable amount of supposition and speculation, with no actual evidence to support his assertions or counter the respondents arguments." The tribunal panel noted that the Commissioner had referred to Professor Anderson's "wild speculations" that "young men, borderline sociopathic or psychopathic" would attempt to identify trial participants from the anonymised data, and said that his views "do him no credit".<ref name="MEAction20160816" /> | ||
The decision noted in the evidence that "Contrast instead Professor Chalder when she accepts that unpleasant things have been said to and about PACE researchers only, but that no threats have been made either to researchers or participants. The highest she could put it was some | The decision noted in the evidence that "Contrast instead Professor Chalder when she accepts that unpleasant things have been said to and about PACE researchers only, but that no threats have been made either to researchers or participants. The highest she could put it was some participants stated that they had been made to feel "uncomfortable" as a result of their contact with and treatment from her, not because of their participation in the trial per se. There is no evidence either of a campaign to identify participants nor even of a risk of an 'insider threat'." | ||
Moreover, regarding the independent [[Cochrane]] review it was admitted in the tribunal that "Professor Chalder states that disclosure to the [[Cochrane]] review does not count as disclosure to independent scientists as all three of the PACE principal investigators sat on the review panel." | Moreover, regarding the independent [[Cochrane]] review it was admitted in the tribunal that "Professor Chalder states that disclosure to the [[Cochrane]] review does not count as disclosure to independent scientists as all three of the PACE principal investigators sat on the review panel." | ||
Additionally, a UK ME sufferer submitted a FOI request in June 2016 and established that the PACE trial investigator's university paid | Additionally, a UK ME sufferer submitted a FOI request in June 2016 and established that the PACE trial investigator's university paid £245,745.27 for legal fees to defend their case in the tribunal against the original ICO decision.<ref>{{Cite web | last = | first = | authorlink = | website = The MEAction Network | title = QMUL spend £250,000 on PACE data tribunal | date = July 9, 2016 | access-date = 2010-10-06 | url = http://www.meaction.net/2016/07/09/qmul-spends-on-pace-tribunal/ }}</ref><ref name="JTJ-public-money">{{Cite web | url = https://johnthejack.com/2016/06/29/using-public-money-to-keep-publicly-funded-data-from-the-public | title = Using public money to keep publicly funded data from the public | first = John | last = Peters | authorlink = John Peters | website = Johnthejack | access-date = October 6, 2022 | date = | ||
September 14, 2016 }}</ref> | |||
The | |||
The lead PACE investigator's university issued a statement the same day stating "We are studying the decision carefully and considering our response".<ref name="QMUL20160816" /> An open letter in support of Alem Matthees was sent to the university and signed by Dr [[Ronald Davis]], Dr [[Jonathan Edwards]], Dr [[Rebecca Goldin]], Dr [[Bruce Levin]], Dr [[Zaher Nahle]], Dr [[Vincent Racaniello]], Dr [[Charles Shepherd]] and Dr [[John Swartzberg]] strongly urging them to not appeal further. | |||
The ME community through [[The MEAction Network | #MEAction]] posted a plea to the PACE trial lead authors to "pursue a complete retraction of their PACE trial paper from the Lancet and all associated subsequent papers from their relevant journals." and to "[e]nd this tragedy now."<ref name="tragedy2016">{{Cite web | website = The MEAction Network | last = | first = | authorlink = The MEAction Network | access-date = October 6, 2022 | url = https://www.meaction.net/2016/09/21/a-plea-for-decency-to-white-chalder-sharpe/ | date = 2016-09-21 | title = A Plea for Decency to White, Chalder & Sharpe }}</ref> QMUL did not appeal and released the data to Matthees.<ref name="QMULnews-noappeal2016">{{ Cite web | url = http://www.qmul.ac.uk/media/news/items/smd/181216.html | publisher = Queen Mary University Of London | access-date = 2016-09-10 | title = Statement: Disclosure of PACE trial data under the Freedom of Information Act | date = September 9, 2016 | url-status = dead | archive-url = http://web.archive.org/web/20160910005225/https://www.qmul.ac.uk/media/news/items/smd/181216.html | archive-date = 2016-09-10 }}</ref> QMUL finally decided not to appeal a second time, and released the PACE trial data.<ref>{{Cite tweet | access-date = October 6, 2022 | date = July 29, 2017 | last = Coyne | first = James | authorlink = James Coyne | url = https://twitter.com/CoyneoftheRealm/status/778643034800005120 | user = CoyneoftheRealm | title = Whoa, sorry, here is the link to the #PACE data 4 all you budding research parasites. Get analyzing. Expose the lies }}</ref> | |||
====Results of Reanalysis of the PACE trial data==== | |||
Virology blog published the re-analysis on September 21, [http://www.virology.ws/2016/09/21/no-recovery-in-pace-trial-new-analysis-finds/ No 'Recovery' in PACE Trial, New Analysis Finds] and concluded "The results should put to rest once and for all any question about whether the PACE trial's enormous mid-trial changes in assessment methods allowed the investigators to report better results than they otherwise would have had. While the answer was obvious from Dr. Tuller's reporting, the new analysis makes the argument incontrovertible." | |||
The full re-analysis was published as ''A preliminary analysis of 'recovery' from chronic fatigue syndrome in the PACE trial using individual participant data'' and was conducted by [[Alem Matthees]], [[Tom Kindlon]], Carly Maryhew, Philip Stark and [[Bruce Levin]] and stated "This re-analysis demonstrates that the previously reported recovery rates were inflated by an average of four-fold.".<ref name="Wilshire,et al, 2017"/> | |||
On August 19, 2016 [[The Centre for Welfare Reform]] also published an update to its earlier 64 page report from April 2016.<ref name="CntrWelfRefm20160819">{{citation | last1 = Centre for Welfare Reform (UK) | title = Major breakthrough on PACE trial | publisher = Centre for Welfare Reform | date = Aug 19, 2016 | url = http://www.centreforwelfarereform.org/news/major-breaktn-pace-trial/00296.html#.V7c_CycXIps.twitter }}</ref> | |||
''A critical commentary and preliminary re-analysis of the PACE trial'' was published in December 2016 in the Journal of '[[Fatigue: Biomedicine, Health & Behavior]]' by Dr [[Carolyn Wilshire]], [[Tom Kindlon]], [[Alem Matthees]] and [[Simon McGrath]] which found that "The claim that patients can recover as a result of CBT and GET is not justified by the data, and is highly misleading to clinicians and patients considering these treatments.<ref name="Wilshire,et al, 2017">{{Cite journal | last1 = Wilshire | first1 = C | authorlink1 = Carolyn Wilshire | last2 = Kindlon | first2 = T | authorlink2 = Tom Kindlon | last3 = Matthees | first3 = A | authorlink3 = Alem Matthees | last4 = McGrath | first4 = S | authorlink4 = Simon McGrath | title = Can patients with chronic fatigue syndrome really recover after graded exercise or cognitive behavioural therapy? A critical commentary and preliminary re-analysis of the PACE trial | journal = Fatigue: Biomedicine, Health & Behavior | volume = 5 | issue = 1 | pages = 43-56 | date = 2017 | doi = 10.1080/21641846.2017.1259724 | url = https://www.researchgate.net/publication/312464313_Can_patients_with_chronic_fatigue_syndrome_really_recover_after_graded_exercise_or_cognitive_behavioural_therapy_A_critical_commentary_and_preliminary_re-analysis_of_the_PACE_trial}}</ref><ref>{{Cite web | website = MEAction | last = | first = | authorlink = The MEAction Network | access-date = October 6, 2022 | url = https://www.meaction.net/2016/12/14/the-pace-trial-where-recovery-doesnt-mean-getting-your-health-back/ | date = 2016-12-14 | title = The PACE Trial: Where "Recovery" Doesn't Mean Getting Your Health Back}}</ref> | |||
'A Rejoinder to Sharpe, Chalder, Johnson, Goldsmith and White' was published by Dr [[Carolyn Wilshire]], [[Tom Kindlon]] and [[Simon McGrath]] in response to the PACE authors reply to their original publication .<ref name="Wilshire C, et al, 2017">{{Cite journal | last1 = Wilshire | first1 = Carolyn | authorlink1 = Carolyn Wilshire | last2 = Kindlon | first2 = T | authorlink2 = Tom Kindlon | last3 = McGrath | first3 = S | authorlink3 = Simon McGrath | title = PACE trial claims of recovery are not justified by the data: a rejoinder to Sharpe, Chalder, Johnson, Goldsmith and White | journal = Fatigue: Biomedicine, Health & Behavior | volume = 5 | issue = 1 | pages = 62-67 | date = 2017 | doi = 10.1080/21641846.2017.1259724 | url = https://www.researchgate.net/publication/315482747_PACE_trial_claims_of_recovery_are_not_justified_by_the_data_A_Rejoinder_to_Sharpe_Chalder_Johnson_Goldsmith_and_White_2017 }}</ref> Wilshire et al disproved again the PACE trials misleading claims as recovery was a strong claim and did not adhere to the core meaning of the word, no evidence that the original protocol specified definition was too stringent and absolute recovery rates from other studies were not legitimate source of support for the recovery definition used. It reinforced the original conclusion that "The PACE trial provides no evidence that CBT and GET can lead to recovery from CFS. The recovery claims made in the PACE trial are therefore misleading for patients and clinicians". | |||
Instead of engaging with critics by releasing other additional requests for data, the PACE trial authors updated their guidelines in January 2017 for those requesting access to the PACE trial data which further restricted access by requiring "formal agreement" with "precise analytic plans, and plans for outputs".<ref>{{Cite web | url = https://www.qmul.ac.uk/wolfson/media/wolfson/current-projects/PACE-Data-Sharing-Policy.pdf | website = Queen Mary University Of London | title = PACE Data Sharing Policy | archive-date = 2019-02-07 | url-status = dead | archive-url = http://web.archive.org/web/20190207044004/https://www.qmul.ac.uk/wolfson/media/wolfson/current-projects/PACE-Data-Sharing-Policy.pdf}}</ref> Prof [[James Coyne]] commented that it is "a ruse to trap the unwary in endless haggling and appeal, while protecting the PACE investigators from the independent reanalysis of the claims, which they have already declared poses a reputational risk to them".<ref name="Coyne20170318" /> | |||
====Scientific and Media response==== | |||
Despite patients and critics not having access to the [[Science Media Centre]] the media coverage especially outside the UK was extensive. For a full list of the scientific and media response to the PACE trial data release and reanalysis, see the article: | |||
* | * [[List of scientific and media response to release of PACE trial data]] | ||
'''Journal of Health Psychology 'Special Issue on the PACE Trial'''' | |||
A special edition of the Journal of Health Psychology was published | A special edition of the Journal of Health Psychology was published on July 31, 2017, by the editor, Dr [[David Marks]] with an editorial - [http://journals.sagepub.com/doi/full/10.1177/1359105317722370 ''Special Issue on the PACE Trial''] and accompanying all [http://journals.sagepub.com/toc/hpqa/22/9 20 Editorials & Commentaries].<ref name="specialissueJHP">{{Cite journal | last = | first = | authorlink = | date = August 2017 | title = Special issue: The PACE Trial | url = http://journals.sagepub.com/toc/hpqa/22/9 | journal = [[Journal of Health Psychology]] | volume = 22 | issue = 9 | pages = | doi = | pmc = | pmid = | quote = | via = }}</ref> | ||
A press release was put out | A press release was put out on July 31, 2017, called ''The PACE Trial: The Making of a Medical Scandal''.<ref name="PACE-scandal-DFM">{{Cite tweet | access-date = October 6, 2022 | last = Marks | first = David F | authorlink = David Marks | date = 2017-07-29 | user = david_f_marks | url = https://twitter.com/david_f_marks/status/891389836954931200 | title = The #PACEtrial team - the only authors to request no peer review. A few red faces on Monday - but will it be anger or shame? }}</ref><ref>{{Cite web | title = The making of the PACE scandal | url = https://citizen-network.org/news/the-making-of-the-pace-scandal | publisher = Centre for Welfare Reform | website = [[Citizen Network]] | access-date = October 6, 2022 | date = 2017-07-28 }}</ref> | ||
Prof Coyne explained in his blog of the [ | Prof Coyne explained in his blog of the "<nowiki>[l]</nowiki>ast ditch attempt to block publication of special issue of Journal of Health Psychology foiled" and that anonymous and powerful PACE proponents made threats to the publisher of JHP, SAGE Publications, that made them reluctant to publish the special edition. explained in his blog "Some threats were made to Sage Publications, the publisher of Journal of Health Psychology, which expressed a reluctance to go forward as planned. As often happens with these kind of pressures, we weren’t told the identity of the complainant. It was clear that whoever s/he was, this person was powerful in being able to grind to a halt of making the special issue available, complete with the introductory editorial that was not previously available.'"<ref name="CoyneBlockAttempt">{{Cite web | last = Coyne | first = James | authorlink = James Coyne | url = https://jcoynester.wordpress.com/2017/07/30/last-ditch-attempt-to-block-publication-of-the-special-issue-of-the-journal-of-health-psychology-foiled/ | date = 2017-07-30 | url-access = registration | title = Last ditch attempt to block publication of special issue of Journal of Health Psychology foiled | access-date = 2022-10-06 }}</ref> | ||
It transpired that "When the effort to block publication of the special issue failed, the PACE investigators got criticism posted at Science Media Centre." <ref>https://jcoynester.wordpress.com/2017/08/01/part-2-what-to-look-for-in-the-special-issue-of-journal-of-health-psychology-concerning-the-pace-trial/ </ref> | It transpired that "When the effort to block publication of the special issue failed, the PACE investigators got criticism posted at Science Media Centre." <ref name="Coyne2017-Part2">{{Cite web | last = Coyne | first = James | authorlink = James Coyne | url-access = registration | website = Quick Thoughts blog | url = https://jcoynester.wordpress.com/2017/08/01/part-2-what-to-look-for-in-the-special-issue-of-journal-of-health-psychology-concerning-the-pace-trial/ | title = | ||
Part 2: What to look for in a Special Issue of Journal of Health Psychology concerning the PACE trial | date = Aug 1, 2017 }}</ref> | |||
The [[Science Media Centre]] put out its own "expert reaction" press release to UK journalists minutes before the special edition was available to spin the story about the PACE scandal and distract and refocus away from the central problems with the scandal.<ref>{{Cite web|url=https://web.archive.org/web/20170731134503/http://www.sciencemediacentre.org/expert-reaction-to-journal-of-health-psychologys-special-issue-on-the-pace-trial/|title= | The [[Science Media Centre]] put out its own "expert reaction" press release to UK journalists minutes before the special edition was available to spin the story about the PACE scandal and distract and refocus away from the central problems with the scandal.<ref name="paceSMC20130131">{{Cite web | archive-url = https://web.archive.org/web/20170731134503/http://www.sciencemediacentre.org/expert-reaction-to-journal-of-health-psychologys-special-issue-on-the-pace-trial/ | url = http://www.sciencemediacentre.org/expert-reaction-to-journal-of-health-psychologys-special-issue-on-the-pace-trial/ | date = Jan 31, 2013 | title = Expert reaction to Journal of Health Psychology's Special Issue on The PACE Trial | website = Science Media Centre | archive-date = 2017-07-31 | url-status = dead }}</ref> The Science Media Centre ignored the glaring problems and instead made personal attacks agains the authors. | ||
Dr [[David Tuller]] | Dr [[David Tuller]] deconstructed "these rather pathetic efforts at defending the indefensible" from the Medical Research Council (MRC), an unnamed University of Oxford spokesperson, and Malcolm Macleod in ''The Science Media Centre's Desperate Efforts to Defend PACE''.<ref>{{Cite web | last1 = Tuller | first1 = David | authorlink1 = David Tuller | title = Trial by Error: The Science Media Centre's Desperate Efforts to Defend PACE | website = [[Virology blog]] | url = http://www.virology.ws/2017/08/02/trial-by-error-the-science-media-centres-desperate-efforts-to-defend-pace/ | date = 2017-08-02 }}</ref> It was stated by a whistleblower that [[Michael Sharpe]] was the person who complained to the Journal to block publication.<ref>{{Cite tweet | access-date = October 6, 2022 | date = August 8, 2016 | last = Peters | first = John | authorlink = John Peters | user = johnthejack | title = According to a whistleblower, the person who complained to Sage prior to publication of the JHP special issue was Prof M Sharpe #PACEtrial | url = https://twitter.com/johnthejack/status/895013220875980800 }}</ref> This was published on social media by a ME advocate and although unverified, due to its importance as it was the principal author of PACE trial, it is being documented and referenced here. | ||
The Times (UK) published two articles | The Times (UK) published two articles that covered the issue but with a spin and focus on disagreements between scientists and resignations rather than the central issues in the Special Issue on the PACE trial scandal.<ref name="Times2017-a">{{Cite news | access-date = Oct 6, 2022 | publisher = The Times | first = Tom | last = Whipple | first2 = Oliver | last2 = Moody | date = Aug 1, 2017 | url = https://www.thetimes.co.uk/edition/news/scientists-trade-insults-over-myalgic-encephalomyelitis-me-study-slk0cv5l | title = Scientists trade insults over myalgic encephalomyelitis (ME) study }}</ref><ref name="Times2017-b">{{Cite news | access-date = Oct 6, 2022 | publisher = The Times | first = Tom | last = Whipple | first2 = Oliver | last2 = Moody | date = Aug 1, 2017 | url = https://www.thetimes.co.uk/article/a-battle-of-prescriptions-99p8sdxs3 | title = A battle of prescriptions }}</ref><ref>{{Cite web | access-date = Oct 6, 2017 | date = Aug 1, 2017 | website = [[ME Association]] | title = The Times: Scientists trade insults over myalgic encephalomyelitis (ME) study {{!}} August 1, 2017 | url = http://www.meassociation.org.uk/2017/08/the-times-scientists-trade-insults-over-myalgic-encephalomyelitis-me-study-01-august-2017/}}</ref><ref>{{Cite web | access-date = Oct 6, 2017 | date = Aug 1, 2017 | last = | first = | authorlink = | website = [[ME Association]] | title = The Times: A battle of prescriptions {{!}} 1 August 2017 | url = http://www.meassociation.org.uk/2017/08/the-times-behind-the-story-a-battle-of-prescriptions-01-august-2017/ }}</ref> The impact of the decades long denial by the PACE authors of the disability could be seen in the Express article ''Is chronic fatigue syndrome real? Life-threatening condition can leave sufferers bed bound''.<ref name="express-838619">{{Cite news | last = | first = | publisher = Daily Express | date = Aug 10, 2017 | url = http://www.express.co.uk/life-style/health/838619/chronic-fatigue-syndrome-real-me-yuppie-flu-symptoms-treatment-cure-controversy | access-date = Oct 6, 2017 | title = Is chronic fatigue syndrome real? {{!}} Life-threatening condition can leave sufferers bed bound | url-status = live}}</ref> The Daily Mail reported on it with ''Angry scientists throw insults at each other over the results of a £5 million taxpayer-funded study into chronic fatigue syndrome''.<ref name="MailAngry">{{Cite web | access-date = 2022-10-06 | publisher = Daily Mail Online | date = August 1, 2017 | last = Burns | first = Jerome | url = http://www.dailymail.co.uk/health/article-4749208/Angry-scientists-throw-insults-regarding-flawed-study.html | title = Angry scientists throw insults at each other over the results of a £5 million taxpayer-funded study into chronic fatigue syndrome }}</ref> | ||
US local media reported on it as [http://www.miningjournal.net/life/2017/08/chronic-fatigue-syndrome-reality-conflicts-with-medical-study/ 'Chronic fatigue syndrome reality conflicts with medical study']. Other international media reporting it including the Metro Netherlands [https://www.metronieuws.nl/lezerscolumn/lou-corsius/actueel/2017/07/wetenschap-valt-door-de-mand 'Wetenschappers vallen door de mand']. | US local media reported on it as [http://www.miningjournal.net/life/2017/08/chronic-fatigue-syndrome-reality-conflicts-with-medical-study/ 'Chronic fatigue syndrome reality conflicts with medical study']. Other international media reporting it including the Metro Netherlands [https://www.metronieuws.nl/lezerscolumn/lou-corsius/actueel/2017/07/wetenschap-valt-door-de-mand 'Wetenschappers vallen door de mand']. Forskning (Research) of Norway reported it as [http://forskning.no/medisin-sykdommer/2017/08/hard-kritikk-av-stor-me-studie#.WYWNpLbhxuE.twitter 'Hard kritikk av stor ME-studie'/'Hard criticism of major ME study'] . Seeker published on both the PACE trial scandal and the 2017 Stanford Cytokine study and concluded "The fact that this new study and the journal issue came out at virtually the same time is significant. The study and the journal "reinforce each other," Tuller said".<ref name="seeker2017">{{Cite news | date = 2017-01-08 | first = Leah | last = Rosenbaum | url = https://www.seeker.com/health/a-new-clue-is-helping-to-solve-the-mystery-behind-chronic-fatigue-syndrome | title = A New Clue Is Helping to Solve the Mystery Behind Chronic Fatigue Syndrome | website = Seeker | access-date = 2022-10-06 }}</ref> | ||
Journalist Jerome Burns in the Daily Mail wrote the article | Journalist Jerome Burns in the Daily Mail wrote the article ''Why are doctors and patients still at war over M.E.? How the best treatment for the debilitating condition is one of the most bitterly contested areas in medicine''.<ref name="MailAngry"/> David Tuller commented on social media "Amazing to see a fair story on ME like this in the UK press. Seems like the PACE story has broken through" as it was a breakthrough in the UK media because of the influence of the PACE authors and the [[Science Media Centre]].<ref>{{Cite tweet | access-date = October 6, 2022 | last = VanElzakker | first = Michael | authorlink = Michael VanElzakker | user = MBVanElzakker | date = Aug 15, 2017 | url = https://twitter.com/MBVanElzakker/status/897337300971081728 | title = Fair-ish. Reminds me of both-sides-ism in climate science. You'd not know from this article that the PACE team are a tiny, isolated faction. }}</ref> | ||
The iNews reported it as | The iNews reported it as ''"You're a disgusting old fart neoliberal hypocrite" – scientists in furious row over ME study'' with a prominent and groundbreaking comment from Dr [[David Marks]] in the national UK media "The many wrongs committed by psychiatry and medicine to the ME/CFS community can only be righted when the Pace trial is ultimately seen for what it is: a disgraceful confidence trick to reduce patient compensation payments and benefits."<ref name="inewsfurious">{{Cite news | url = https://inews.co.uk/essentials/news/health/scientists-furious-row-pace-trial-journal-special-edition/ | title = 'You’re a disgusting old fart neoliberal hypocrite' – scientists in furious row over ME study' | publisher = iNews | date = August 1, 2017 | access-date = 2022-10-06 }}</ref> | ||
The Medical Independent in Ireland published an article referencing Dr [[Keith Geraghty]] and [[Nasim Marie Jafry]]'s | The Medical Independent in Ireland published an article referencing Dr [[Keith Geraghty]] and [[Nasim Marie Jafry]]'s work with a literary take on the changing narrative of ME from neurological to biopsychosocial and culminating in the PACE trial and a whole special issue dedicated to the scandal.<ref>{{Cite news | url = http://www.medicalindependent.ie/100315/is_it_just_me_or_is_this_a_real_disease | title = Is it just ME or is this a real disease? | archive-date = 2017-10-23 | archive-url = http://web.archive.org/web/20171023203008/http://www.medicalindependent.ie:80/100315/is_it_just_me_or_is_this_a_real_disease | first = George | last = Winter | date = Oct 16, 2017 | website = Medical Independent | access-date = 2022-10-06 | url-status = dead }}</ref> | ||
The President of the [[IACFS/ME]] Prof [[Fred Friedberg]] stated of the Special Edition "the most misleading and damaging aspect of the PACE trial was contained in the subsequent reporting of | The President of the [[IACFS/ME]] Prof [[Fred Friedberg]] stated of the Special Edition "the most misleading and damaging aspect of the PACE trial was contained in the subsequent reporting of "recovery"" and "This can have the effect of delegitimizing the illness even more and may discourage biomedical research on ME/CFS". He continued "misleading reports that inflated the benefits of the PACE trial, which were widely cited in the mass media at the time, may have further undermined illness credibility with the research community" as far as the United States.<ref>{{Cite journal | via = TwitLonger | volume = 10 | issue = 2 | date = April 2017 | journal = IACFS/ME Newsletter | title = Adverse Impact on Research of the #PACETrial? | first = Fred | last = Friedberg | authorlink = Fred Friedberg }}</ref> In contrast a separate investigation into research funding it was established Peter White as receiving the most research funding and a near monopoly from the UK government and taxpayers out of all researchers despite protests from patients.<ref name="MEA-funding2016">{{Cite web | access-date = 2010-10-06 | website = [[ME Association]] | title = Research Funding {{!}} An Overview Of Activity By Major Instutional Funders Included On The Dimensions Database | first = Giles | last = Radford | first2 = Sonya | last2 = Chowdhury | authorlink2 = Sonya Chowdhury | date = 2016 | url = http://www.meassociation.org.uk/wp-content/uploads/mecfs-research-funding-report-2016.pdf }}</ref> | ||
'''Learn more''' | |||
* 2018, [http://parliamentlive.tv/event/index/cf2fde9d-f327-4bf4-8e72-1fc6124b8998?in = 11:01:20&out = 11:31:59 UK Parliament debate on ME Treatment] - UK Parliament (video), [https://hansard.parliament.uk/commons/2018-02-20/debates/990746C7-9010-4566-940D-249F5026FF73/PACETrialPeopleWithME Hansard transcript] | |||
*2018, [https://www.youtube.com/watch?v=UwCEvnXZTlA PACE trial and its effect on people with ME (alternate video #2] - UK Parliament via YouTube | |||
*2018, [https://www.youtube.com/watch?v=XpVfZqcjeVY PACE trial and its effect on people with ME (alternate video #3] - UK Parliament via YouTube | |||
*2018, [https://www.youtube.com/watch?v=frhFmr7rbzc PACE trial and its effect on people with ME (alternate video #4] - UK Parliament via YouTube | |||
*2016, [https://www.statnews.com/2016/09/21/chronic-fatigue-syndrome-pace-trial/ Bad science misled millions with chronic fatigue syndrome. Here’s how we fought back], [[Julie Rehmeyer]], ''Stat News'', 21 | * 2017, [http://journals.sagepub.com/doi/full/10.1177/1359105317722370 'Special Issue on the PACE Trial'] - The Journal of Health Psychology | ||
*2016, [https://www.statnews.com/2016/09/21/chronic-fatigue-syndrome-pace-trial/ Bad science misled millions with chronic fatigue syndrome. Here’s how we fought back], [[Julie Rehmeyer]], ''Stat News'', Sep 21, 2016.<ref name="Rehmeyer20160921" /> | |||
== Reaction by Scientific, Medical and ME Communities== | ===Reaction by Scientific, Medical and ME Communities=== | ||
The PACE trial was scientifically discredited but the impact of it on the medical community has continued especially in the UK. | The PACE trial was scientifically discredited but the impact of it on the medical community has continued especially in the UK. | ||
'''Agency for Healthcare Research and Quality (AHRQ) amendment, US''' | |||
The US government agency called the [[Agency for Healthcare Research and Quality]] (AHRQ) revised its earlier recommendations about found that when studies of CBT and GET, including PACE, that used the Oxford | The US government agency called the [[Agency for Healthcare Research and Quality]] (AHRQ) revised its earlier recommendations about found that when studies of CBT and GET, including PACE, that used the [[Oxford criteria]] for CFS were excluded, there was no evidence for graded exercise and only weak evidence for CBT.<ref name="ahrq-change">{{cite web | last1 = Dimmock | first1 = Mary | authorlink1 = Mary Dimmock | first2 = Jennie | last2 = Spotila | authorlink2 = Jennie Spotila | title = AHRQ agrees: GET useless, CBT ineffective | website = Occupy ME | date = Aug 18, 2016 | url = http://occupyme.net/2016/08/16/ahrq-evidence-review-changes-its-conclusions/ }}</ref> [[Mary Dimmock]] led the way on this issue.<ref name="ahrq-change"/> | ||
'''PLOS Expression of Concern''' | |||
Prof James Coyne had requested anonymised data in 2015 from the PACE trial authors from the Cost Effectiveness paper. He pursued this request throughout 2015 and 2016 but was also refused as "vexatious". After 18 months, | Prof James Coyne had requested anonymised data in 2015 from the PACE trial authors from the Cost Effectiveness paper. He pursued this request throughout 2015 and 2016 but was also refused as "vexatious". After 18 months, PLOS issued an Expression of Concern on May 2nd 2017. It stated "We conclude that the lack of resolution towards release of the dataset is not in line with the journal's editorial policy and we are thus issuing this Expression of Concern to alert readers about the concerns raised about this article.<ref>{{Cite journal | last = The PLOS ONE Editors | first = | date = 2017-05-02 | title = Expression of Concern: Adaptive Pacing, Cognitive Behaviour Therapy, Graded Exercise, and Specialist Medical Care for Chronic Fatigue Syndrome: A Cost-Effectiveness Analysis | url = https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0177037 | journal = PLOS ONE | volume | ||
= 12 | issue = 5 | pages = e0177037 | doi = 10.1371/journal.pone.0177037 | issn = 1932-6203 | pmc = 5412692 | pmid = 28463341}}</ref> The editors of PLOS One, Iratxe Puebla and Joerg Heber, wrote in PLOS One Blog "Since we feel we have exhausted the options to make the data available responsibly, and considering the questions that were raised about the validity of the article's conclusions, we have decided to post an Expression of Concern to alert readers that the data are not available in line with the journal's editorial policy".<ref>{{Cite web | url = https://everyone.plos.org/2017/05/02/data-sharing-in-clinical-research/ | title = Data sharing in clinical research: challenges and open opportunities | last = | first = | authorlink = | date = 2017-05-02 | website = PLoS | language = en-US | archive-url = | archive-date = | url-status = | access-date = 2020-07-19}}</ref> Retraction Watch reported on the development.<ref>{{Cite web | url = http://retractionwatch.com/2017/05/02/plos-upgrades-flag-controversial-pace-chronic-fatigue-syndrome-trial-authors-surprised/ | date = 2017-05-02 | website = Retraction watch | title = PLOS upgrades flag on controversial PACE chronic fatigue syndrome trial; authors "surprised" }}</ref> | |||
'''Centers for Disease Control & Prevention (CDC), US''' | |||
David Tuller reported | David Tuller reported in July 2017 that the [[Centers for Disease Control and Prevention | CDC]] had updated their treatment recommendations and that all mention of CBT and GET as treatment or management strategies had "disappeared". Tuller reported that the key members of the PACE trial had a long history with the CDC and "For advocates, the CDC's removal of the CBT/GET recommendations represents a major victory. The removal of CBT and GET has been "heralded as an important" by patient charities.<ref>{{cite web | last = ME Association | authorlink1 = ME Association | url = http://www.meassociation.org.uk/2017/07/cdc-removes-cbt-and-get-as-recommended-treatments-for-mecfs-11-july-2017/ | date = Jul 11, 2017 | website = [[ME Association]] | title = CDC removes CBT and GET as recommended treatments for ME/CFS {{!}} 11 July 2017 }}</ref><ref>{{cite web | url = https://undark.org/2017/07/26/cdc-chronic-fatigue-graded-exercise/ | last = Tuller | first = David | authorlink = David Tuller | website = Undark | title = CDC Removes Reference to Disputed ME/CFS Therapies From Website | date = 2017-07-26 }}</ref> | ||
In September, [[David Tuller]] and [[Julie Rehmeyer]] wrote an article in STAT exploring [https://www.statnews.com/2017/09/25/chronic-fatigue-syndrome-cdc/ | In September, [[David Tuller]] and [[Julie Rehmeyer]] wrote an article in STAT exploring ''Why did it take the] [[CDC]] so long to reverse course on debunked treatments for chronic fatigue syndrome?''.<ref name="STAT2017">{{Cite news | last = Rehmeyer | first1 = Julie | authorlink1 = Julie Rehmeyer | last2 = Tuller | first2 = David | authorlink2 = David Tuller | publisher = STAT | url = https://www.statnews.com/2017/09/25/chronic-fatigue-syndrome-cdc/ | title = Why did it take the] CDC so long to reverse course on debunked treatments for chronic fatigue syndrome? | date = 2017-09-05 }}</ref> NPR reported on the change in the CDC recommendations and that exercise can make the disease worse and criticised the seminal paper Sir [[Simon Wessely]] from 1989 that led to exercise as a treatment and the PACE trial.<ref>{{Cite news | url = http://www.npr.org/sections/health-shots/2017/10/02/554369327/for-people-with-chronic-fatigue-syndrome-more-exercise-isnt-better | publisher = NPR | date = Oct 2, 2017 | title = For People With Chronic Fatigue Syndrome, More Exercise Isn't Better }}</ref> In November 2017, the New York Times published an article stating that longstanding advice to exercise was now recognised as not only ineffective but counterproductive, and referred to the CDC and [[NICE guidelines review | NICE decision to review the recommendations]].<ref>{{Cite news | url = https://www.nytimes.com/2017/11/27/well/new-recognition-for-chronic-fatigue.html | date = 2017-11-12 | publisher = New York Times | first = Jane E. | last = Brody | title = New Recognition for Chronic Fatigue}}</ref> | ||
'''UK reaction from the Journals - Lancet, Psychological Medicine and Guideline Provider - NICE''' | |||
The UK medical and scientific establishment have been obstinate in changing their position to reflect the flaws of the PACE trial as the PACE trial investigators are highly influential and senior members of the UK establishment. The Lancet have refused to retract the study as yet. Psychological Medicine have also refused to retract the study as well. | The UK medical and scientific establishment have been obstinate in changing their position to reflect the flaws of the PACE trial as the PACE trial investigators are highly influential and senior members of the UK establishment. The Lancet have refused to retract the study as yet. Psychological Medicine have also refused to retract the study as well. | ||
The controversial [[NICE guidelines]] were challenged by patient groups after the PACE trial was discredited by way of petitions and challenges by ME patient groups in 2017. | The controversial [[NICE guidelines]] were challenged by patient groups after the PACE trial was discredited by way of petitions and challenges by ME patient groups in 2017. ME sufferers have taken other actions due to the UK medical establishment intransigence of acknowledging the issues. The Medical Research Council (MRC) and its Chief Executive continued to defend the trial and in a statement dated August 28, 2018 said that as funders of the PACE trial we reject the view that the scientific evidence provided by the trial was unsound.<ref>{{Cite web | url = https://mrc.ukri.org/news/browse/criticism-of-the-pace-trial/ | title = Criticism of the PACE trial | last = Medical Research Council | first = | date = 2018-08-28 | website = mrc.ukri.org | language = en | access-date = 2019-04-09}}</ref> | ||
Jerome Burns in Health Insights UK published a follow up article to his 2016 articles on the PACE trial in the article headlined | Jerome Burns in Health Insights UK published a follow up article to his 2016 articles on the PACE trial in the article headlined 'The claim that the cure for the crippling fatigue of ME/CFS was to change your mind always seemed bizarre. Now it really is on the way out…' The journalist stated he thought it would be big story in 2016 with lots of repercussions but the UK press largely ignored it and he revisited the story in 2019 due to professional opinions changing against CBT/GET, with the recent House of Commons debates and the impending revision of the NICE guidelines.<ref>{{Cite web | url = http://healthinsightuk.org/2019/04/22/the-claim-that-the-cure-for-the-crippling-fatigue-of-mecfs-was-to-change-your-mind-always-seemed-bizarre-now-it-really-is-on-the-way-out/ | title = The claim that the cure for the crippling fatigue of ME/CFS was to change your mind always seemed bizarre. Now it really is on the way out… | date = 2019-04-22 | website = HealthInsightUK | language = en-US | access-date = 2019-04-23 }}</ref> | ||
'''Continued campaigning''' | |||
[[Mary Dimmock]] wrote a comprehensive report | [[Mary Dimmock]] wrote a comprehensive report ''Clinical Guidance for ME: “Evidence-Based” Guidance Gone Awry'' in January 2018 that details how the PACE trial and similar studies based on flawed science led to evidence-based reviews and clinical guidance recommending harmful treatments for ME patients.<ref>{{Cite web | first = Mary | last = Dimmock | authorlink = Mary Dimmock | url = https://www.dropbox.com/s/kbjmmuc1utx67md/Concerns%20with%20ME%20Evidence%20Based%20Guidance%20January%202018.pdf?dl=0 | title = Clinical Guidance for ME: "Evidence-Based" Guidance Gone Awry | date = January 2018 }}</ref><ref>{{Cite web | title = The Failure of Clinical Guidance for People with ME | website = The MEAction Network | last = | first = | authorlink = The MEAction Network | access-date = October 6, 2022 | url = https://www.meaction.net/2018/02/05/the-failure-of-clinical-guidance-for-people-with-me/ | date = 2018-02-05 }}</ref> | ||
ME sufferers | ME sufferers campaigned against the PACE trial in the [[Millions Missing protests]] around the world in 2018, and about the adverse effects of the trial on on biomedical research. As part of this a 'Song for ME: Blowin in the Wind' (available on [https://www.youtube.com/watch?v=BncUDXRA1v4 YouTube]) was recorded by ME patients from 7 countries around the world which included the lyrics "How many times must an idea fail...Before it is seen to be flawed?...And how many flaws can a trial embrace...Before it is seen as a fraud?...Yes and how many wounds must its victims expose... | ||
Before they’re no longer ignored?" <ref>https://www.meaction.net/2018/04/24/a-song-for-me-blowin-in-the-wind/</ref> | Before they’re no longer ignored?".<ref>{{Cite web | url = https://www.meaction.net/2018/04/24/a-song-for-me-blowin-in-the-wind/ | website = The MEAction Network | last = | first = | authorlink = The MEAction Network | access-date = October 6, 2022 | date = 2018-04-24 | title = A song for ME: Blowin' in the Wind }}</ref> | ||
Dr Racaniello sent another open letter with nearly 100 signatories including scientists, clinicians, academics, lawyers | Dr Racaniello sent another open letter with nearly 100 signatories including scientists, clinicians, academics, lawyers and other experts to the editor Richard Horton at the Lancet due to receiving no response two years later to the original letter from February 2016. The letter again asked for an independent re-analysis from independent reviewers outside UK psychiatry who have no conflicts of interests involving the PACE investigators and the funders of the trial.<ref>{{Cite web | last = Tuller | first = David | authorlink = David Tuller | website = [[Virology blog]] | url = http://www.virology.ws/2018/06/19/trial-by-error-an-open-letter-to-the-lancet-two-years-on/ | date = Jun 19, 2018 | title = Trial By Error: An Open Letter to The Lancet, Two Years On }}</ref> Another letter of appeal was sent with signatories including 65 ME patient organisation / charities and also this time with 10 Members of Parliament signing the open letter to Richard Horton and the Lancet.<ref>{{Cite web | last = Tuller | first = David | authorlink = David Tuller | website = [[Virology blog]] | url = http://www.virology.ws/2018/07/10/trial-by-error-yet-another-appeal-to-the-lancet-with-more-on-board/ | date = Jul 10, 2018 | title = Trial By Error: Yet Another Appeal to The Lancet, With More On Board }}</ref> The Times (UK) reported on the third letter in its article ''Call for review of 'flawed' ME research in Lancet letter'' and stated at the end, "The Lancet declined to comment".<ref name="flawed-me-research-Times">{{Cite news | publisher = The Times | url = https://www.thetimes.co.uk/article/call-for-review-of-flawed-me-research-in-lancet-letter-l75rvcprh | title = The Times: Call for review of 'flawed' ME research in Lancet letter | date = August 21, 2018 | access-date = 2019-04-09}}</ref> The BMJ who were supportive of the PACE trial previously reported it as 'Pressure grows on Lancet to review “flawed” PACE trial' and also approached the Lancet for comment.<ref>{{citation | last1 = Torjesen | first1 = Ingrid | title = Tackling fears about exercise is important for ME treatment, analysis indicates | journal = BMJ | page = 350 | date = 2015 | url = https://www.bmj.com/content/362/bmj.k3621.short }}</ref> In a letter to the editor of The Times, the Chief Executive of the MRC, Fiona Watt continued to defend the PACE trial.<ref name="flawed-me-research-Times"/><ref>{{Cite news | url = https://www.thetimes.co.uk/article/preparing-children-for-starting-primary-primary-school-6q6hv8nl3 | title = Preparing children for starting primary school | date = 2018-08-27 | access-date = 2019-04-09 | language = en | issn = 0140-0460}}</ref> | ||
=== Additional Scientific Publications=== | ====Additional Scientific Publications==== | ||
=====BMC Psychology - Re-analysis by Wilshire et al===== | |||
BMC Psychology published on | BMC Psychology published on March 22nd, 2018 ''Rethinking the treatment of chronic fatigue syndrome—A reanalysis and evaluation of findings from a recent major trial of graded exercise and CBT'' by [[Carolyn Wilshire]], [[David Tuller]], [[Tom Kindlon]], [[Alem Matthees]], [[Keith Geraghty]], [[Robert Courtney]] and [[Bruce Levin]].<ref name="Wilshire2018"/> It concluded these findings raise serious concerns about not only the PACE trial but the robustness of the claims made about the efficacy of CBT and GET as a whole. One of the early critics and author, [[Robert Courtney]], died shortly before the paper was published.<ref name="Wilshire2018">{{Cite journal | last = Wilshire | first = Carolyn E. | authorlink = Carolyn Wilshire | last2 = Kindlon | first2 = Tom | author-link2 = Tom Kindlon | last3 = Courtney | first3 = Robert | author-link3 = Robert Courtney | last4 = Matthees | first4 = Alem | author-link4 = Alem Matthees | last5 = Tuller | first5 = David | author-link5 = David Tuller | last6 = Geraghty | first6 = Keith | author-link6 = Keith Geraghty | last7 = Levin | first7 = Bruce | author-link7 = Bruce Levin | date = Mar 22, 2018 | title = Rethinking the treatment of chronic fatigue syndrome-a reanalysis and evaluation of findings from a recent major trial of graded exercise and CBT | url = https://www.ncbi.nlm.nih.gov/pubmed/29562932 | journal = BMC psychology | volume = 6 | issue = 1 | pages = 6 | doi = 10.1186/s40359-018-0218-3 | issn = 2050-7283 | pmc = 5863477 | pmid = 29562932 | quote = | via = }}</ref> | ||
Once again the [[Science Media Centre]] on the eve of the publication organised media briefings for journalists and news organisations | Once again, the [[Science Media Centre]] on the eve of the publication organised three media briefings for journalists and news organisations, and published a CFS/ME factsheet plus two briefings in what was seen as an attempt to reduce the impact of the publication and spin the story.<ref>{{Cite web | url = http://www.sciencemediacentre.org/wp-content/uploads/2018/03/Science-Media-Centre-Factsheet-CFS-ME-Final-Version.pdf | title = Factsheet: CFS/ME – The illness and the controversy | last = Science Media Centre | authorlink = Science Media Centre | website = [[Science Media Centre]] | date = Mar 2018}}</ref><ref>{{Cite web | url = http://www.sciencemediacentre.org/reanalysis-of-the-pace-trial/ | title = Expert reaction to reanalysis of the PACE trial for chronic fatigue syndrome (CFS) treatments | last = Science Media Centre | authorlink = Science Media Centre | website = [[Science Media Centre]] | date = Mar 2018}}</ref> One of the patient authors of the PACE reanalysis asked other patients to contact journalists to ensure they cover the publication and to ensure they have the full facts.<ref>{{Cite web | website = [[Science for ME]] | url-status = dead | title = Science media centre CFS/ME factsheet for journalists: the illness and the controversy | url = https://www.s4me.info/threads/science-media-centre-cfs-me-factsheet-for-journalists-the-illness-and-the-controversy.3104/ | date = 2018 }}</ref> Despite the pressure and Science Media Centre briefings, the media did report on PACE trial reanalysis. ''The Times'' reported it as ''Findings of £5m ME chronic fatigue study 'worthless'''. Even the publicy-funded news organisation, the BBC, reported on it with ''Chronic fatigue trial results 'not robust', new study says''. The Daily Mail, Huffington Post and other UK national and local media reported on the story.<ref>{{Cite news | url = http://www.dailymail.co.uk/wires/pa/article-5532233/Findings-chronic-fatigue-study-not-reliable.html | publisher = Daily Mail | title = Before the headlines analysis – Reanalysis of the PACE trial | date = Mar 2018 }}</ref><ref>{{Cite news | url = https://www.huffingtonpost.co.uk/2018/03/22/findings-of-chronic-fatigue-study-not-reliable_n_19401888.html | publisher = Huffington Post | date = Mar 22, 2018 | title = Findings Of Chronic Fatigue Study 'Not Reliable' }}</ref><ref>{{Cite web | website = [[ME Association]] | url = http://www.meassociation.org.uk/2018/03/reanalysis-of-the-pace-trial-finds-impressive-claims-for-recovery-following-cbt-and-get-are-not-statistically-reliable-22-march-2018/ | date = Mar 22, 2018 | title = ME Association Press Release: Reanalysis of the PACE trial finds impressive claims are 'not statistically reliable' {{!}} 22 March 2018}}</ref> The news was reported in Europe by Health Europa.<ref>{{Cite web | url = https://www.healtheuropa.com/controversy-me-pace-trial/85888/ | date = May 8, 2018 | title = The controversy surrounding the 'unreliable' ME PACE trial }}</ref> The Canary's [[Steve Topple]] published in-depth coverage of the reanalysis paper.<ref>{{Cite news | url = https://www.thecanary.co/discovery/analysis-discovery/2018/03/22/the-mainstream-medical-community-just-declared-war-on-people-living-with-me/ | publisher = The Canary | first = Steve | last = Topple | authorlink = | date = 2018-03-22 | title = The mainstream medical community just declared war on people living with ME }}</ref> The NEJM JWatch publicised this to physicians.<ref>{{Citation | url = https://www.jwatch.org/na46472/2018/04/06/chronic-fatigue-treatment-redux-questioning-efficacy-cbt | date = 2018-04-06 | website = NEJM JWatch | title = Chronic Fatigue Treatment Redux: Questioning the Efficacy of CBT and Graded Exercise }}</ref> The European Health Journal also republished the paper.<ref name="repubEHJ">{{citation | url = https://www.europeanhealthjournal.com/2018/04/26/rethinking-the-treatment-of-chronic-fatigue-syndrome-a-reanalysis-and-evaluation-of-findings-from-a-recent-major-trial-of-graded-exercise-and-cbt/ | website = | via = European Health Journal | date = 2018-04-26 | title = Rethinking the treatment of chronic fatigue syndrome—A reanalysis and evaluation of findings from a recent major trial of graded exercise and CBT }}</ref> | ||
In their correspondence to BMC Psychology published on March 12, 2019 PACE trial authors [[Michael Sharpe]], [[Kimberley Goldsmith]] and [[Trudie Chalder]] responded to the re-analysis by Wilshire et al, with ''The PACE trial of treatments for chronic fatigue syndrome: a response to Wilshire et al''. They restated that "both CBT and GET, when given appropriately as supplements to specialist medical care, are more effective at improving both fatigue and physical functioning in people with CFS, than are APT or SMC alone." and that they found "no good reason" to change the conclusions of the PACE trial.<ref = "BMCPsych-response2019">{{Cite journal | last = Sharpe | first = Michael | authorlink = Michael Sharpe | last2 = Goldsmith | first2 = Kim | author-link2 = Kimberley Goldsmith | last3 = Chalder | first3 = Trudie | author-link3 = Trudie Chalder | author-link4 = | authorlink5 = | date = Mar 12, 2019 | title = The PACE trial of treatments for chronic fatigue syndrome: a response to WILSHIRE et al | url = https://doi.org/10.1186/s40359-019-0288-x | journal = BMC Psychology | volume = 7 | issue = 1 | pages = 15 | doi = 10.1186/s40359-019-0288-x | issn = 2050-7283 | quote = | via = }}</ref> Wilshire and Kindlon also responded in BMC Psychology with 'Response: Sharpe, Goldsmith and Chalder fail to restore confidence in the PACE trial findings''.<ref>{{Cite journal | last = Wilshire | first = Carolyn E. | last2 = Kindlon | first2 = Tom | date = 2019-03-26 | title = Response: Sharpe, Goldsmith and Chalder fail to restore confidence in the PACE trial findings | url = https://doi.org/10.1186/s40359-019-0296-x | journal = BMC Psychology | volume = 7 | issue = 1 | pages = 19 | doi = 10.1186/s40359-019-0296-x | issn = 2050-7283 | pmc = | pmid = 30914065}}</ref> In the rejoinder Wilshire and Kindlon clarify the misconception in the PACE trial author’s commentary, and address the seven additional arguments they raise in defence of their conclusion. They concluded "New arguments presented by Sharpe et al. inspire some interesting reflections on the scientific process, but they fail to restore confidence in the PACE trial’s original conclusions." It was also stated that [[research bias in ME/CFS | unjustified optimism of CBT/GET]] fuelled by the PACE trial has caused many consequences including hindering the search for effective treatments and that the debate around the PACE trial will have implications reaching far and beyond. | |||
=====Other journals===== | |||
Also in March 2018, the British Journal of Healthcare Management published a summary of the controversy over the decades and the scandal over the last two years.<ref>{{citation | url = https://www.magonlinelibrary.com/doi/abs/10.12968/bjhc.2018.24.3.112 | title = Chronic fatigue syndrome: the ongoing medical debate | journal = Journal of Healthcare Management | volume = 24 | issue = 3 | pages = | first = George | last = Winter | date = Mar 10, 2018 }}</ref> | |||
'' | In July 2018, scientists from the [[Workwell Foundation]], University of the Pacific, California, published an editorial in the British Journal of Sports Medicine ''Checking our blind spots: current status of research evidence summaries in ME/CFS'', and concluded "the UK PACE Trial has exposed some of the most important public health-related ethical and legal challenges of our time, which, as yet, remain largely under-reported and unknown to many clinicians." It was supported citizen scientists in its conclusion "We should follow the example of engaged patient advocates and citizen scientists in ME/CFS by involving patients as advisory board members on trials and as reviewers on published evidence summaries....By including evidence from patients in clinical decisions and research, we can begin to address the important blind spots in how we think about and implement EBP."<ref name="blind-spots">{{Cite journal | last = Davenport | first = Todd E | authorlink = Todd Davenport | last2 = Stevens | first2 = Staci R | author-link2 = Staci Stevens | last3 = VanNess | first3 = J Mark | author-link3 = Mark VanNess | last4 = Stevens | first4 = Jared | author-link4 = | last5 = Snell | first5 = Christopher R | author-link5 = Christopher Snell | date = Oct 2019 | title = Checking our blind spots: current status of research evidence summaries in ME/CFS | url = http://bjsm.bmj.com/lookup/doi/10.1136/bjsports-2018-099553 | journal = British Journal of Sports Medicine | language = en | volume = 53 | issue = 19 | pages = 1198–1198 | doi = 10.1136/bjsports-2018-099553 | issn = 0306-3674 | pmc = | pmid = | access-date = | quote = | via = }}</ref> | ||
=====<span id="#PACE-Gate">'PACE-Gate': When clinical trial evidence meets open data access</span>===== | |||
In | In November 2016, [[Keith Geraghty]] published an editorial in the Journal of Health Psychology describing the PACE trial controversy.<ref name="PACE-Gate-Geraghty2016"/> Geraghty stated: | ||
:"Patients claim the lead authors overstated the effectiveness of cognitive behavioural therapy and graded exercise therapy by lowering the thresholds they used to determine improvement. In this extraordinary case, patients discovered that the treatments tested had much lower efficacy after an information tribunal ordered the release of data from the PACE trial to a patient who had requested access using a freedom of information request".<ref name="PACE-Gate-Geraghty2016">{{Cite journal | url = https://journals.sagepub.com/doi/10.1177/1359105316675213?icid=int.sj-full-text.similar-articles.2 | title = 'PACE-Gate': When clinical trial evidence meets open data access | first = Keith J | last = Geraghty | authorlink = Keith Geraghty | date = November 1, 2016 | journal = Journal of Health Psychology | volume = 22 | issue = 9 | pages = 1106-1112 | doi = 10.1177/1359105316675213 | pmid = }}</ref> | |||
'' | * Nov 1, 2016, [https://journals.sagepub.com/doi/10.1177/1359105316675213 'PACE-Gate': When clinical trial evidence meets open data access] - Keith Geraghty | ||
=====Textbooks===== | |||
In March 2018 'Health Psychology: Theory, Research and Practice' by Dr David Marks et al documented the numerous flaws in the PACE trial in a section called 'The PACE trial: A Catalogue of Errors' | In March 2018, the book 'Health Psychology: Theory, Research and Practice' by Dr [[David Marks]] et al. documented the numerous flaws in the PACE trial in a section called ''The PACE trial: A Catalogue of Errors''.<ref name="PACE-trial-errors">{{Cite book | chapter = Long-term Conditions: Diabetes and ME/CFS | pages = 483-505 | chapter-url = https://books.google.co.uk/books?id=FlXqDwAAQBAJ&pg=PA500&newbks=1&newbks_redir=0 | title = Health Psychology: Theory, Research and Practice | editor-last = Marks | editor-first = David | editor-link1 = David Marks | editor-last2 = Murray | editor-first2 = Michael | editor-last3 = Estacio | editor-first3 = Emee Vida | date = 2020 | publisher = SAGE | isbn = 1529736153 | edition = 6 }}</ref> | ||
In August 2018 | In August 2018 the book ''Psychology in Crisis'' by Prof Brian Michael Hughes also documented and summarised the failures of the PACE trial.<ref>{{Cite book | first = Brian Michael | last = Hughes | authorlink = Brian Hughes | title = Psychology in Crisis | date = 2018 | isbn = 9781352003017 | publisher = Macmillan Education UK | url = https://books.google.co.uk/books?id=2B1HEAAAQBAJ&pg=PA139&dq=PACE+trial+inauthor:hughes&hl=en&newbks=1&newbks_redir=0 }}</ref> | ||
===Complaint to medical regulator (GMC)=== | =====Complaint to UK medical regulator (GMC)===== | ||
Dr Sarah Myhill has complained to the UK's medical regulator, the [ | Dr Sarah Myhill has complained to the UK's medical regulator, the [[General Medical Council]], over the conduct of the PACE trial authors.<ref>{{cite web | last1 = Myhill | first1 = Sarah | authorlink1 = Sarah Myhill | title = My Complaint to the GMC about the PACE trial authors | website = Dr Myhill | date = 2021 | url = http://www.drmyhill.co.uk/wiki/My_Complaint_to_the_GMC_about_the_PACE_authors | url-status = dead | archive-url = http://web.archive.org/web/20210418162823/https://www.drmyhill.co.uk/wiki/My_Complaint_to_the_GMC_about_the_PACE_authors | archive-date = 2021-04-18 }}</ref> Over 200 letters of complaints by ME patients were also sent to the General Medical Council. A petition gathered over 8,900 signatories supporting Dr Myhill's complaint to the GMC about the PACE trial authors.<ref>{{Cite web | url = https://www.change.org/p/the-general-medical-council-i-am-showing-my-support-for-dr-myhill-s-complaint-to-the-gmc-about-the-pace-authors | website = change.org | title = I am showing my support for Dr Myhill's complaint to the GMC about the PACE authors | date = | last = Robinson | first = Craig | authorlink = Craig Robinson }}</ref> | ||
===Further FOIA requests=== | =====Further FOIA requests===== | ||
Further Freedom of Information Act 2000 requests have been made to the PACE trial team at [[Queen Mary University of London]] (QMUL). The PACE authors | Further Freedom of Information Act 2000 requests have been made to the PACE trial team at [[Queen Mary University of London]] (QMUL), including a request for a copy of the minutes of the PACE trial management group meetings.<ref name="ICO-meetings-decision2018"/> The PACE authors continued to refuse to release the PACE trial meeting minutes and other data using various reasons including refusing an internal review and these decisions were appealed again to the Information Commissioner's Office. On February 20, 2018 the Information Commissioner's Office ruled that the the university should release the [[PACE Trial Steering Committee]] minutes and the [[PACE Trial Management Group | Trial Management Group]] minutes. The PACE team and the university surprisingly repeated its earlier arguments to the ICO that the criticism is unwarranted and the trial is not controversial among the majority of scientists in the field and that there is a history of the researchers being personal abused, their integrity being questioned and even as far as saying they may risk threats of physical violence. The Information Tribunal in August 2016 in its court decision rejected these serious but unfounded assertions. The complainant, an ME sufferer, argued that this data should be released given the previous submissions by the PACE authors were unreliable and in the intervening period the scientific community in addition to patients had cast further concerns about the trial. The Commissioner found that the public interest favors publishing the anonymized information and ruled that the university should release the minutes of the PACE trial meetings, and had 35 days to do so.<ref name="ICO-meetings-decision2018">{{Cite web | url = https://ico.org.uk/media/action-weve-taken/decision-notices/2018/2258289/fs50687719.pdf | website = Information Commissioner's Office | title = Freedom of Information Act 2000 (FOIA) Decision notice {{!}} Queen Mary University of London Mile End Road {{!}} Reference: FS50687719 | date = February 20, 2018 }}</ref> | ||
=== Second Parliamentary debate on PACE Trial=== | ====Second Parliamentary debate on PACE Trial==== | ||
It was announced on 6 | It was announced on February 6, 2018 by [[Carol Monaghan]] MP that a Westminster Hall debate would be held on the PACE trial called 'PACE trial and its effect on people with ME'.<ref>{{Cite web | first = Carol | last = Monaghan | authorlink = Carol Monaghan | url = http://www.carol.monaghan.scot/2018/02/07/pace-trial/ | title = SNP MP secures debate on controversial DWP-funded ME trial | date = February 6, 2018 }}</ref> This was exactly five years to the date of the last PACE trial debate in 2013.<ref>{{Cite news | publisher = The Herald | url = http://www.heraldscotland.com/news/15982563.Glasgow_MP_secures_parliamentary_examination_of_controversial_ME_trial/ | title = Glasgow MP Carol Monaghan secures parliamentary examination of controversial ME trial | date = 2018}}</ref> | ||
The 'PACE Trial and its effect on people with ME' debate took place on 20 | The ''PACE Trial and its effect on people with ME'' debate took place on February 20, 2018 at Westminster Hall.<ref name="UKParl20180220-transcript">{{cite web | author1 = UK Parliament | title = PACE trial and its effect on people with ME (transcript) | date = Feb 20, 2018 | url = https://hansard.parliament.uk/commons/2018-02-20/debates/990746C7-9010-4566-940D-249F5026FF73/PACETrialPeopleWithME }}</ref> The debate was concluded by Carol Monaghan MP "I think that when the full details of the trial become known, it will be considered one of the biggest medical scandals of the 21st century."<ref name="UKParl20180220-transcript"/> | ||
ME charities [[Invest in ME Research]], ME Association and others commented on the debate.<ref>{{Cite web | website = Invest in ME Research | last = Invest in ME Research | authorlink = Invest in ME Research | url = http://www.investinme.org/IIMER-Newslet-1802-02.shtml | title = SNP MP secures debate on controversial DWP-funded ME trial | date = February 2018 }}</ref><ref>{{ Cite web | website = [[ME Association]] | title = Government-funded ME/CFS trial 'one of greatest medical scandals of 21st century' {{!}} 20 February 2018 | url = http://www.meassociation.org.uk/2018/02/government-funded-me-cfs-trial-one-of-greatest-medical-scandals-of-21st-century-20-february-2018/ | date = February 20, 2018 }}</ref> | |||
ME | ME advocates have produced an analysis of Caroline Dinenage MP (Department of Health and Social Care) who responded on behalf of the government with mixed and inconsistent responses but continued support for the PACE trial..<ref>{{Cite web | website = The MEAction Network | last = | first = | authorlink = The MEAction Network | access-date = October 6, 2022 | title = Westminster Hall Debate on the PACE trial and its effects on people with ME | url = https://www.meaction.net/2018/02/26/read-about-the-westminster-hall-debate-on-the-pace-trial-and-its-effects-on-people-with-me/ | date = February 27, 2018 }}</ref> | ||
ME | An Early Day Motion (EDM) 1247 was launched by Carol Monaghan MP in May for ME Awareness week which stated amongst other points "That this House recognises Myalgic Encephalomyelitis (ME) Awareness Week from 6 to 12 May 2018....acknowledges the detrimental effect of the PACE trial and its results, and the work which is being done to reverse this".<ref>{{Cite web | url = https://www.parliament.uk/edm/2017-19/1247 | title = ME AWARENESS WEEK 2018 - Early Day Motions | publisher = UK Parliament | date = 2018}}</ref> It was supported by 136 MPs and was in the top 1% (No 7/1503 as at July 2018) of all EDM's in the parliamentary session. | ||
A further debate on 'M.E. Treatment and Research' was led by Carol Monaghan MP on 21 June 2018 and the PACE trial was again [[UK_Parliament_Grand_Committee_Room_debate_21st_June_2018#The_PACE_trial | criticised by many MPs]]. | |||
===Quotes from PACE trial critics=== | |||
The PACE trial received extensive criticism from a range of experts. For a selection of quotes from critics about the PACE trial, see the article: | |||
* [[Quotes from critics of the PACE trial]] | |||
===Principal investigators and researchers=== | |||
The principal investigators on the PACE trial are Professors [[Peter White | Peter D. White]], [[Trudie Chalder]] and [[Michael Sharpe | Michael Sharpe]]. Additional authors on the 2011 Lancet paper of the [[PACE Trial Management Group]] are [[Kimberley Goldsmith]], [[Anthony Johnson | Anthony L. Johnson]], [[Laura Potts]], [[Rebecca Walwyn]], [[Julia DeCesare]], [[Hannah Baber | Hannah L. Baber]], [[Mary Burgess]], [[Lucy Clark | Lucy V. Clark]], [[Diane Cox | Diane L. Cox]], [[Jessica Bavinton]], [[Brian Angus]], [[Gabrielle Murphy]], [[Maurice Murphy]], [[Hazel O'Dowd]], [[David Wilks]], and [[Paul McCrone]]. | |||
The [[PACE Trial Steering Committee]] members included [[Mansel Aylward]]. | |||
===List of PACE trial publications=== | |||
'''2003: Trial Registry. BioMed Central. ISRCTN54285094''' | |||
< | There is a currently published trial register and there is a version of the register that is no longer available on the web but has been archived.<ref name="pace2003trialregistry">{{cite journal | last1 = White | first1 = PD | authorlink1 = Peter White | title = Trial Registry ISRCTN54285094 | journal = BioMed Central | date = May 22, 2003 | url = http://www.isrctn.com/ISRCTN54285094}}</ref><ref name="pace2003trialregistryarchive">{{cite journal | last1 = White | first1 = PD | authorlink1 = Peter White | title = Trial Registry (Archive) ISRCTN54285094 | journal = BioMed Central | date = 2003 | url = http://web.archive.org/web/20050524130106/http://www.controlled-trials.com/mrct/trial/CHRONIC%20FATIGUE%20SYNDROME/1042/40645.html}}</ref> Both versions contain slightly different details. The archived version contains the details of the trial's pre-specified endpoint analyses. | ||
'''2006: Final protocol version 5.0''' | |||
This full version of the study protocol, which includes trial materials such as questionnaires and consent forms, was never officially published.<ref name="pace2006protocolv5">{{citation | last1 = PACE Trial Management Group | authorlink1 = PACE Trial Management Group | title = PACE Trial Protocol: Final Version 5.0 | date = Feb 1, 2006 | url = http://www.meactionuk.org.uk/FULL-Protocol-SEARCHABLE-version.pdf}}</ref> | |||
'''2007: PACE trial protocol''' | |||
[[BMC Neurology]] published the trial's planned methodology, including its planned analyses.<ref name="pace2007protocol">{{citation | last1 = White | first1 = PD | authorlink1 = Peter White | last2 = Sharpe | first2 = MC | authorlink2 = Michael Sharpe | last3 = Chalder | first3 = T | authorlink3 = Trudie Chalder | last4 = DeCesare | first4 = JC | authorlink4 = Julia DeCesare | last5 = Walwyn | first5 = R | authorlink5 = Rebecca Walwyn | last6 = The PACE trial group | authorlink6 = PACE trial group | title = Protocol for the PACE Trial: A randomised controlled trial of adaptive pacing, cognitive behaviour therapy, and graded exercise as supplements to standardised specialist medical care versus standardised specialist medical care alone for patients with the chronic fatigue syndrome/myalgic encephalomyelitis or encephalopathy | journal = BMC Neurology | date = Mar 8, 2007 | pmid = 17397525 | doi = 10.1186/1471-2377-7-6 | url = http://bmcneurol.biomedcentral.com/articles/10.1186/1471-2377-7-6}}</ref> | |||
'''2011: Main trial outcomes''' | |||
The trial's main outcomes, and some selected secondary outcomes, were reported in [[The Lancet]].<ref name="pace2011a" /> Forty-four letters were submitted in a response that the journal's editor described as "swift and damning".<ref name="pace2011LancetEd" /> | |||
===2011: | The [[Science Media Centre]] organised a press briefing and Expert Opinion on ME/CFS Study, on Feb 17, 2011.<ref name="paceSMC20110217" /> The UK and international media heavily publicised this paper after the PACE authors' press conference and in conjunction with the Science Media Centre in February 2011.<ref name="Guardian20110218" /><wbr><ref name="Reuters20110217" /><ref name="WebMD20110217" /><ref name="BBCUK20110218" /><ref name="CNNnews20110218" /><wbr><ref name="NYT20110218">{{cite news | last1 = New York Times | date = Feb 17, 2011 | title = Psychotherapy Eases Chronic Fatigue Syndrome, Study Finds | url = http://www.nytimes.com/2011/02/18/health/research/18fatigue.html }}</ref><ref name="Fox2011">{{Cite news | url = http://www.foxnews.com/health/2011/02/18/pushing-limits-help-chronic-fatigue-patients.html | publisher = Fox news | title = Pushing Limits Can Help Chronic Fatigue Patients | date = 2011-02-18 }}</ref><wbr><ref name="SkyArchived">{{Cite web | publisher = Sky News | date = Feb 18, 2011 | title = Talking And Exercise Could Cure ME - Study | first = Thomas | last = Moore | url = http://news.sky.com/skynews/Home/UK-News/Chronic-Fatigue-Syndrome-ME-Could-Be-Reversed-With-Counselling-And-Exercise-Research-Suggests/Article/201102315935405 | archive-date = 2011-02-18 | archive-url = https://web.archive.org/web/20110221183606/http://news.sky.com/skynews/Home/UK-News/Chronic-Fatigue-Syndrome-ME-Could-Be-Reversed-With-Counselling-And-Exercise-Research-Suggests/Article/201102315935405?lpos=UK_News_Second_Home_Page_Article_Teaser_Region_0&lid=ARTICLE_15935405_Chronic_Fatigue_Syndrome_ME_Could_Be_Reversed_With_Counselling_And_Exercise,_Research_Suggests | url-status = dead}}</ref><ref>{{Cite news | url = https://www.theguardian.com/society/2011/feb/18/study-exercise-therapy-me-treatment | publisher = The Guardian | title = Study finds therapy and exercise best for ME | date = 2011-02-18 }}</ref><ref>{{Cite news | url = http://www.netdoctor.co.uk/healthy-eating/news/a20654/counselling-and-exercise-appear-best-for-me/ | publisher = Net Doctor | title = Counselling and exercise appear best for ME | date = February 17, 2011 }}</ref><ref>{{Cite news | url = https://www.express.co.uk/news/uk/229817/Trial-offers-hope-for-ME-sufferers | title = Trial offers hope for ME sufferers | date = Feb 18, 2011 | publisher = Daily Express | archive-date = Oct 21, 2020 | archive-url = http://web.archive.org/web/20190815121206/https://www.express.co.uk/news/uk/229817/Trial-offers-hope-for-ME-sufferers }} </ref><ref>{{Cite news | url = http://www.independent.co.uk/life-style/health-and-families/health-news/got-me-just-get-out-and-exercise-say-scientists-2218377.html | title = Got ME? Just get out and exercise, say scientists | date = 2011-02-18 | publisher = The Independent }}</ref><wbr><ref>{{Cite web | url = http://www.webmd.boots.com/news/20110217/me-cfs-pacing-yourself-isnt-the-answer | website = Boots | archive-url = http://web.archive.org/web/20150911121457/http://www.webmd.boots.com/news/20110217/me-cfs-pacing-yourself-isnt-the-answer | title = ME/CFS: 'Pacing yourself isn’t the answer' | first = Nicky | last = Broyd | archive-date = 2015-09-11 | url-status = dead | date = 2011-02-17 }}</ref><ref>{{Cite web | url = http://www.mirror.co.uk/lifestyle/sex-relationships/could-new-techniques-ease-living-117621 | title = Could new techniques ease living with ME? | first = Miriam | last = Stoppard | date = Sep 27, 2022 | publisher = The Mirror | access-date = 2022-10-05 }}</ref><ref>{{Cite news | url-status = dead | archive-date = 2011-02-23 | url = http://www.itv.com/news/new-me-breakthrough57275/ | archive-url = https://web.archive.org/web/20110223231102/http://www.itv.com/news/new-me-breakthrough57275/ | date = Feb 18, 2011 | publisher = ITV News | title = Exercise 'helps ME' }}</ref><ref>{{cite news | url = http://www.huffingtonpost.com/jacob-teitelbaum-md/pace-study-may-as-well-sa_b_833621.html | title = To What Extent Can The Mind Heal The Body? | publisher = Huffington Post | first = Jacob | last = Teitelbaum | authorlink = Jacob Teitelbaum | date = Mar 13, 2011 }}</ref><ref name="BBCUK20110218">{{cite news | publisher = BBC News | title = Brain and body training treats ME, UK study says | date = Feb 18, 2011 | url = http://www.bbc.com/news/health-12493009 }}</ref><ref>{{cite news | url = http://www.scotsman.com/news/me-sufferers-given-hope-as-scottish-team-leads-way-to-new-therapy-1-1501751 | publisher = The Scotsman | date = 2011-04-14 | title = Is exercise always good for the body? }}</ref><wbr><ref>{{cite news | url = http://www.scotsman.com/lifestyle/is-exercise-always-good-for-the-body-1-1589145 | publisher = The Scotsman | date = 2011 | title = ME sufferers given hope as Scottish team leads way to new therapy | url-status = dead }}</ref><ref name="CF2011">{{Cite web | archive-url = https://web.archive.org/web/20110221092107/http://www.cbc.ca/news/health/story/2011/02/18/chronic-fatigue-exercise-behaviour-therapy.html | archive-date = 2011-02-21 | url = https://web.archive.org/web/20110221092107/http://www.cbc.ca/news/health/story/2011/02/18/chronic-fatigue-exercise-behaviour-therapy.html | title = Chronic fatigue may be reversed with exercise - Health | publisher = CBC News | location = Canada | date = }}</ref><ref>{{Cite news | url = http://www.msnbc.msn.com/id/41651311/ns/health-health_care/#.UNHy5uTtRXM | publisher = MSNBC | title = For chronic fatigue syndrome, , rest may not be best | first = Maria | last = Cheng | date = 2011-02-17 | url-status = dead }}</ref> | ||
The | |||
'''2012: Cost-effectiveness of CBT and GET''' | |||
This paper was published in the [[PLoS One]] journal and concluded that [[cognitive behavioral therapy]] and [[graded exercise therapy]] had the best probability of being the most cost-effective treatments.<ref name="pace2012CE" /> The UK media publicised the results from this paper<ref>{{Cite news | url = http://www.bbc.co.uk/news/health-19076398 | publisher = BBC News | title = Chronic fatigue syndrome: Brain training is most cost-effective treatment | date = August 2, 2012 }}</ref><ref>{{cite news | url = http://www.nursingtimes.net/nursing...fatigue-syndrome/5047819.article | website = Nursing Times | title = NICE backs CBT for chronic fatigue syndrome | date = Aug 2, 2012 | last = THE PRESS ASSOCIATION}}</ref><ref>{{Cite news | url = http://www.independent.co.uk/life-style/health-and-families/health-news/two-chronic-fatigue-syndrome-treatments-offer-good-value-8000997.html | publisher = The Independent | title = Two chronic fatigue syndrome treatments offer good value | date = August 2, 2012 }}</ref><ref>{{Cite news | url = http://www.huffingtonpost.co.uk/2012/08/02/how-to-spot-symptoms-chronic-fatigue-syndrome_n_1732004.html | publisher = Huffington Post | title = Never Feel Refreshed By Sleep? How To Spot Chronic Fatigue Syndrome | date = 2012-08-02 }}</ref><ref>{{Cite web | website = [[National Health Service]] | url = http://www.nhs.uk/news/2012/08august/Pages/Pacing-for-chronic-fatigue-syndrome-not-cost-effective.aspx | date = Aug 8, 2012 | archive-url = http://web.archive.org/web/20120804061329/http://www.nhs.uk/news/2012/08august/Pages/Pacing-for-chronic-fatigue-syndrome-not-cost-effective.aspx | url-status = dead | archive-date = 2012-08-04 | title = Pacing 'not cost-effective’ for CFS }}</ref> Controversy has broken out over the failure of the study authors to provide the underlying data to Professor James Coyne under PLoS One's data-sharing policy.<ref name="Coyne20160214" /> Journalist [[David Tuller]] has criticized the study's assumptions and conclusions.<ref name="Tuller20151222" /> | |||
<ref name=" | |||
<ref | |||
<ref>http://www. | |||
<ref> | |||
<ref>http://www. | |||
<ref> | |||
'''2013: "Recovery" rates''' | |||
This paper appeared in [[Psychological Medicine]].<ref name="pace2013a" /> The [[Science Media Centre]] issued an "expert opinion" about the study.<ref name="paceSMC20130131"/> | |||
This paper appeared in [[Psychological Medicine]].<ref name="pace2013a" /> The [[Science Media Centre]] issued an "expert opinion" about the study.<ref name="paceSMC20130131" /> | |||
ME/CFS patient [[Graham McPhee]] and others created a video animation - [https://www.youtube.com/watch?v=d_7J5ELjArU How's that recovery?] - explaining problems with the new analyses that had replaced those specified in the study protocol. [[Sam Carter]] applied the new fatigue and physical function recovery criteria to the data from the [[FINE trial]] and found that doing so increased the number of "recovered" patients six-fold, compared to the original criteria.<ref name="CarterSam20160215pubmed" /> | ME/CFS patient [[Graham McPhee]] and others created a video animation - [https://www.youtube.com/watch?v=d_7J5ELjArU How's that recovery?] - explaining problems with the new analyses that had replaced those specified in the study protocol. [[Sam Carter]] applied the new fatigue and physical function recovery criteria to the data from the [[FINE trial]] and found that doing so increased the number of "recovered" patients six-fold, compared to the original criteria.<ref name="CarterSam20160215pubmed" /> | ||
| Line 883: | Line 595: | ||
Patient-advocate [[Peter Kemp]] also criticized the study, stating that the authors had "twisted the SF-36 Physical Function subscale to suit their needs."<ref name="KempPet20160217" /> | Patient-advocate [[Peter Kemp]] also criticized the study, stating that the authors had "twisted the SF-36 Physical Function subscale to suit their needs."<ref name="KempPet20160217" /> | ||
Patients created a tongue-in-cheek song video to satirically criticize the recovery paper results.<ref name="MERecoverySong" /> | Patients created a tongue-in-cheek song video to satirically criticize the recovery paper results.<ref name="MERecoverySong">{{cite web | last1 = ME Analysis | title = ME Recovery Song | type = video | date = Apr 27, 2014 | url = https://www.youtube.com/watch?v=QbKTBMyZfX0}}</ref> | ||
'''2013: Statistical analysis plan''' | |||
Trials Journal published the detailed plan for PACE's data analysis.<ref name="pace2013sap" /> | Trials Journal published the detailed plan for PACE's data analysis.<ref name="pace2013sap" /> | ||
'''2014: Adverse effects''' | |||
The Journal of Psychosomatic Research published the results on safety and adverse effects.<ref name="pace2014AE" /> | The Journal of Psychosomatic Research published the results on safety and adverse effects.<ref name="pace2014AE" /> | ||
'''2015: Secondary mediation analysis''' | |||
[[The Lancet Psychiatry]] published this paper. <ref name="pace2015SMA" /> | |||
<ref>http://www.economist.com/news/science-and-technology/21639438-controversial-trial-mysterious-disease-continues-yield-insights-fear </ref> | [[The Lancet Psychiatry]] published this paper. <ref name="pace2015SMA" /> The UK and international media publicised the results from this paper.<ref name="FoxNews20150114" /><ref name="Guardian20150114" /><ref>{{Cite news | publisher = BBC News | title = Exercise can help with ME, scientists say | url = http://www.bbc.co.uk/news/health-30795506 | date = Jan 14, 2015 }}</ref><ref>{{cite news | url = http://www.economist.com/news/science-and-technology/21639438-controversial-trial-mysterious-disease-continues-yield-insights-fear | publisher = The Economist | title = Fear to tread | date = 2015-01-15 }}</ref><ref>{{Cite news | publisher = Daily Mail | title = Chronic fatigue victims fear exercise could aggravate their condition | date = January 13, 2015 | url = http://www.dailymail.co.uk/health/article-2909029/Chronic-fatigue-victims-suffer-fear-exercise-anxious-activities-aggravate-condition.html }}</ref><ref>{{Cite news | url = http://www.independent.co.uk/life-style/health-and-families/health-news/sufferers-of-chronic-fatigue-syndrome-can-benefit-from-exercise-9976254.html | publisher = The Independent | title = Chronic fatigue syndrome sufferers 'can benefit from exercise'. There is now a consensus that CFS, which affects 250,000 in the UK, is real. | first = Charlie | last = Cooper | date = Jan 14, 2015 | quote = "...evidence of the benefits of graded exercise therapy, which involves a gradual increase in physical activity, remains contentious, with some CFS patients saying it brought them no benefit" }}</ref><ref name="Telegraph20151028" /> | ||
<ref>http://www.dailymail.co.uk/health/article-2909029/Chronic-fatigue-victims-suffer-fear-exercise-anxious-activities-aggravate-condition.html </ref> | <ref>{{cite journal | last = Torjesen | first = Ingrid | title = Tackling fears about exercise is important for ME treatment, analysis indicates | volume = 350 | pages = h227 | doi = 10.1136/bmj.h227 | date = January 14, 2015 | journal = BMJ | url = http://www.bmj.com/content/350/bmj.h227 }}</ref><ref>{{Cite web | url = https://www.sciencedaily.com/releases/2015/01/150113204351.htm | website = Science Daily | title = Reducing fear avoidance beliefs key to improving symptoms and reducing disability in chronic fatigue syndrome | date = Jan 2015 }}</ref><ref>{{Cite web | url = http://www.nhs.uk/news/2015/01January/Pages/Therapy-and-exercise-may-help-some-with-CFS.aspx | date = 2015-01-01 | website = [[National Health Service]] | title = Therapy and exercise may help some with CFS }}</ref><ref>{{Cite web | url = https://medicalxpress.com/news/2015-01-beliefs-key-symptoms-disability-chronic.html | date = Jan 2015 | website = Medical Express | title = Reducing fear avoidance beliefs key to improving symptoms and reducing disability in chronic fatigue syndrome }}</ref> | ||
<ref>http://www.independent.co.uk/life-style/health-and-families/health-news/sufferers-of-chronic-fatigue-syndrome-can-benefit-from-exercise-9976254.html | |||
<ref>http://www.bmj.com/content/350/bmj.h227 </ref> | |||
<ref>https://www.sciencedaily.com/releases/2015/01/150113204351.htm </ref> | |||
<ref>http://www.nhs.uk/news/2015/01January/Pages/Therapy-and-exercise-may-help-some-with-CFS.aspx</ref> | |||
<ref>https://medicalxpress.com/news/2015-01-beliefs-key-symptoms-disability-chronic.html | |||
The paper has been criticised by [[Byron Hyde]].<ref name="HydeByr20150122ftm" /> | The paper has been criticised by [[Byron Hyde]].<ref name="HydeByr20150122ftm" /> | ||
[[File:Lancet_pace_follow-up_study_chart.jpg|100px|thumb|right|PACE long-term follow-up]] | [[File:Lancet_pace_follow-up_study_chart.jpg | 100px | thumb | right | PACE long-term follow-up]] | ||
'''2015: Long-term follow-up''' | |||
Long-term follow-up results were published in [[The Lancet Psychiatry]] in October 2015.<ref name="pace2015FU" /> | Long-term follow-up results were published in [[The Lancet Psychiatry]] in October 2015.<ref name="pace2015FU" /> | ||
| Line 916: | Line 622: | ||
The [[Science Media Centre]] published "expert reaction" to the paper.<ref name="paceSMC20151028" /> The UK media then publicised the results. | The [[Science Media Centre]] published "expert reaction" to the paper.<ref name="paceSMC20151028" /> The UK media then publicised the results. | ||
<ref name="EveningStdUK20151028" /><ref name="IndepIE20151028" /><ref name="Torjesen20151028" /><ref name="DailyMail20151028" /><ref name="Telegraph20151028" /><ref name="ScienceMag20151027" /> | <ref name="EveningStdUK20151028" /><ref name="IndepIE20151028" /><ref name="Torjesen20151028" /><ref name="DailyMail20151028" /><ref name="Telegraph20151028" /><ref name="ScienceMag20151027" /> | ||
<ref>http://www.bbc.co.uk/programmes/p036fnz0 | <ref name="Radio4-2015">{{Citation | title = Today, 28/10/2015, 'Best treatment' for Chronic Fatigue Syndrome | website = BBC Radio 4 | date = 2015-10-28 | access-date = | ||
October 6, 2022 | url = http://www.bbc.co.uk/programmes/p036fnz0 }}</ref><ref>{{Cite news | url = https://uk.news.yahoo.com/positive-thinking-exercise-could-help-091553348.html | title = Positive Thinking And Exercise Could Help Beat Chronic Fatigue Syndrome, Study Suggests | date = Oct 28, 2015 }}</ref> | |||
Professors [[James Coyne]] and [[Keith Laws]] criticised the results as "uninterpretable" and consisting of "null findings."<ref name="Coyne20151029mb" /> The [[ME Association]] also criticized the follow-up study.<ref name="MEASSUK20151028press" /> | Professors [[James Coyne]] and [[Keith Laws]] criticised the results as "uninterpretable" and consisting of "null findings."<ref name="Coyne20151029mb" /> The [[ME Association]] also criticized the follow-up study.<ref name="MEASSUK20151028press" /> | ||
'''2015: Longitudinal mediation analysis''' | |||
This short paper published in Trials Journal concluded: "Approximately half of the effect of each of CBT and GET [...] on physical function was mediated through reducing avoidance of fearful situations".<ref name="pace2015lm" /> | This short paper published in Trials Journal concluded: "Approximately half of the effect of each of CBT and GET [...] on physical function was mediated through reducing avoidance of fearful situations".<ref name="pace2015lm" /> | ||
===Other publications=== | ====Other publications==== | ||
*2014: [https://www.ncbi.nlm.nih.gov/pubmed/23967878 Pain in chronic fatigue syndrome: response to rehabilitative treatments in the PACE trial] | *2014: [https://www.ncbi.nlm.nih.gov/pubmed/23967878 Pain in chronic fatigue syndrome: response to rehabilitative treatments in the PACE trial] | ||
*2013: [http://andrewgelman.com/wp-content/uploads/2016/01/White-Psych-Bulletin-2015.pdf The planning, implementation and publication of a complex intervention trial for chronic fatigue syndrome: the PACE trial]<ref name="PACEplanning2013">{{Cite journal|last=Sharpe|first=Michael| | *2013: [http://andrewgelman.com/wp-content/uploads/2016/01/White-Psych-Bulletin-2015.pdf The planning, implementation and publication of a complex intervention trial for chronic fatigue syndrome: the PACE trial]<ref name="PACEplanning2013">{{Cite journal | last = Sharpe | first = Michael | authorlink = Michael Sharpe | last2 = Chalder | first2 = Trudie | author-link2 = Trudie Chalder | last3 = White | first3 = Peter D. | author-link3 = Peter White | author-link4 = | authorlink5 = | authorlink6 = | date = Feb 2015 | title = The planning, implementation and publication of a complex intervention trial for chronic fatigue syndrome: the PACE trial | url = https://www.cambridge.org/core/journals/bjpsych-bulletin/article/planning-implementation-and-publication-of-a-complex-intervention-trial-for-chronic-fatigue-syndrome-the-pace-trial/539F6F7132848EE05207A4E271813C9A/core-reader | journal = BJPsych Bulletin | language = en | volume = 39 | issue = 1 | pages = 24–27 | doi = 10.1192/pb.bp.113.045005 | issn = 2056-4694 | quote = | via = }}</ref> | ||
*2013: [https://www.ncbi.nlm.nih.gov/pubmed/22571806 Cognitions, behaviours and co-morbid psychiatric diagnoses in patients with chronic fatigue syndrome] | *2013: [https://www.ncbi.nlm.nih.gov/pubmed/22571806 Cognitions, behaviours and co-morbid psychiatric diagnoses in patients with chronic fatigue syndrome] | ||
*2013: [https://www.researchgate.net/publication/257763666_Training_Supervision_Therapists'_Adherence_to_Manual_Based_Therapies Training, Supervision & Therapists' Adherence to Manual Based Therapies] | *2013: [https://www.researchgate.net/publication/257763666_Training_Supervision_Therapists'_Adherence_to_Manual_Based_Therapies Training, Supervision & Therapists' Adherence to Manual Based Therapies] | ||
*2011: [https://www.ncbi.nlm.nih.gov/pubmed/?term=21843745 Measuring disability in patients with chronic fatigue syndrome: reliability and validity of the Work and Social Adjustment Scale] | *2011: [https://www.ncbi.nlm.nih.gov/pubmed/?term = 21843745 Measuring disability in patients with chronic fatigue syndrome: reliability and validity of the Work and Social Adjustment Scale] | ||
*2010: [https://www.ncbi.nlm.nih.gov/pubmed/21103120 Psychiatric misdiagnoses in patients with chronic fatigue syndrome] | *2010: [https://www.ncbi.nlm.nih.gov/pubmed/21103120 Psychiatric misdiagnoses in patients with chronic fatigue syndrome] | ||
==Talks and interviews== | ===Talks and interviews=== | ||
*2011, [https://www.youtube.com/watch?v=U0_8eVl99zs Michael Sharpe interview] | *2011, [https://www.youtube.com/watch?v=U0_8eVl99zs Michael Sharpe interview] | ||
*2011: Professors Michael Sharpe and Trudie Chalder were videoed<ref name="pace2011press1" /><ref name="pace2011press2" /><ref name="pace2011press3" /> at [[The Lancet]]'s press conference for the publication of the trial's main results in 2011. | *2011: Professors Michael Sharpe and Trudie Chalder were videoed<ref name="pace2011press1" /><ref name="pace2011press2" /><ref name="pace2011press3" /> at [[The Lancet]]'s press conference for the publication of the trial's main results in 2011. | ||
*2011: | *2011: "Health Report - Comparison of treatments for chronic fatigue syndrome - the PACE trial", ABC Radio National (Australia), 18 Apr 2011<ref name="pace20110418radio" /> | ||
*2015: Professor James Coyne gave a talk on "A skeptical look at the PACE chronic fatigue trial" in Edinburgh | *2015: Professor James Coyne gave a talk on "A skeptical look at the PACE chronic fatigue trial" in Edinburgh,<ref name="Coyne20151116-v1" /><ref name="Coyne20151116-v2" /><ref name="Coyne20151116-v3" /> transcript,<ref name="Coyne20151116-tl" /> and slides<ref name="Coyne20151116-ss" /> available online) and, more recently, Belfast<ref name="Coyne20160208-v" /> and slides <ref name="Coyne20160208-ss" /> | ||
*2016: While in Amsterdam at The [[Forgotten Plague]] Conference, [[David Tuller]] gave an [https://www.youtube.com/watch?v=UN1bYhkDHA4&feature=youtu.be '''interview'''] | *2016: While in Amsterdam at The [[Forgotten Plague]] Conference, [[David Tuller]] gave an [https://www.youtube.com/watch?v=UN1bYhkDHA4&feature = youtu.be '''interview'''] with [[Frank Twisk]] on 27 Feb 2016.<ref name="Tuller20160227-v" /> The following day, Feb 28, 2016, David Tuller gave a [https://www.youtube.com/watch?v=1fS6Gzc52VI '''speech'''] with Q&As.<ref name="Tuller20160228-v" /> | ||
*June 2017: [https://www.youtube.com/watch?v=6zWnVB_2lUU&frags=pl%2Cwn #TearItUp PACE trial] Invest in ME Pre-Conference dinner talk by Dr David Tuller | *June 2017: [https://www.youtube.com/watch?v=6zWnVB_2lUU&frags = pl%2Cwn #TearItUp PACE trial] Invest in ME Pre-Conference dinner talk by Dr David Tuller | ||
*September 2018: | *September 2018: [https://www.youtube.com/watch?v=PRCQ-kIxQyY How Not To Conduct A Randomized Clinical Trial] by Dr [[Bruce Levin]], Ph.D., Department of Biostatistics, Mailman School of Public Health, Columbia University Irving Medical Center | ||
*October 2018: [https://www.facebook.com/Hope4MEFibro/videos/vb.1003993319659488/549092118889078/?type=2&theater 'The PACE Trial: ‘One of Greatest Medical Scandals of the 21st Century'] talk by Dr David Tuller and Professor Brian Hughes, Newry, Northern Ireland | *October 2018: [https://www.facebook.com/Hope4MEFibro/videos/vb.1003993319659488/549092118889078/?type=2&theater 'The PACE Trial: ‘One of Greatest Medical Scandals of the 21st Century'] talk by Dr David Tuller and Professor Brian Hughes, Newry, Northern Ireland | ||
==See also== | ===See also=== | ||
*[[PACE trial documents]] | *[[PACE trial documents]] | ||
*[[Intimidation and bullying of PACE trial critics]] | *[[Intimidation and bullying of PACE trial critics]] | ||
*[[Open letter to the Lancet]] | |||
*[[An open letter to Psychological Medicine about “recovery” and the PACE trial]] | |||
*[[List of scientific and media response to release of PACE trial data]] | |||
*[[Action for ME]] | *[[Action for ME]] | ||
*[[Cochrane]] | *[[Cochrane]] | ||
*[[Cognitive behavioral therapy]] | *[[Cognitive behavioral therapy]] | ||
*[[Graded exercise therapy]] | *[[Graded exercise therapy]] | ||
*[[FINE trial]] | *[[FINE trial]] - PACE's "sister trial" | ||
*[[Journal of Health Psychology, Special Issue: The PACE Trial]] | *[[Journal of Health Psychology, Special Issue: The PACE Trial]] | ||
*[[Quotes from critics of the PACE trial]] | |||
*[[NICE guidelines]] | *[[NICE guidelines]] | ||
*[[PACE Trial Management Group]] | *[[PACE Trial Management Group]] | ||
*[[PACE Trial Steering Committee]] | *[[PACE Trial Steering Committee]] | ||
*[[:Category:PACE trial critics|PACE trial critics]] | *[[Research bias in ME/CFS]] | ||
*[[:Category:Psychological paradigm proponents|Proponents of the psychological theory of ME/CFS]] | *[[:Category:PACE trial critics | PACE trial critics]] | ||
*[[:Category:Psychological paradigm critics|Psychological paradigm critics]] | *[[:Category:Psychological paradigm proponents | Proponents of the psychological theory of ME/CFS]] | ||
*[[:Category:Psychological paradigm critics | Psychological paradigm critics]] | |||
*[[The Lancet]] | *[[The Lancet]] | ||
*[[The Lancet Psychiatry]] | *[[The Lancet Psychiatry]] | ||
==Learn more== | ===Learn more=== | ||
* Twitter # : [https://twitter.com/search?f=tweets&q=pacetrial&src=typd #PACEtrial] [https://twitter.com/search?f=tweets&q=pacegate&src=typd #PACEgate] | * Twitter # : [https://twitter.com/search?f=tweets&q=pacetrial&src=typd #PACEtrial] [https://twitter.com/search?f=tweets&q=pacegate&src=typd #PACEgate] | ||
| Line 967: | Line 679: | ||
* Phoenix Rising Forum: [http://forums.phoenixrising.me/index.php?search/43411490/&q=pace+trial&t=post&o=date&g=1 PACE trial] | * Phoenix Rising Forum: [http://forums.phoenixrising.me/index.php?search/43411490/&q=pace+trial&t=post&o=date&g=1 PACE trial] | ||
* Science for ME Forum: [https://www.s4me.info/search/228403/?q=pace+trial&o=date PACE trial] | * Science for ME Forum: [https://www.s4me.info/search/228403/?q=pace+trial&o=date PACE trial] | ||
*[https:// | *[https://www.qmul.ac.uk/wiph/centres/centre-for-psychiatry-and-mental-health/research/pace-trial/ Pace Trial Website] | ||
==References== | ===References=== | ||
<!-- citations for PACE publications, in date order --- --- --- --- ---> | <!-- citations for PACE publications, in date order --- --- --- --- ---> | ||
<references> | <references> | ||
<ref name="pace20060331theft">{{citation | last1 = White | first1 = PD | authorlink1 = Peter White | title = Letter from Professor Peter White re Theft of patient data | date = Mar 31, 2006 | url = https://dl.dropboxusercontent.com/u/23608059/PDW-re-theft.pdf}}</ref> | |||
<ref name="pace2011press1">{{citation | last1 = Sharpe | first1 = M | authorlink1 = Michael Sharpe | last2 = Chalder | first2 = T | authorlink2 = Trudie Chalder | title = London Press Conference: Chronic Fatigue Syndrome - part 1 (video) | journal = The Lancet TV | date = Feb 17, 2011 | url = https://www.youtube.com/watch?v=MR16NQhHdik }}</ref> | |||
<ref name="pace2011press2">{{citation | last1 = Sharpe | first1 = M | authorlink1 = Michael Sharpe | title = London Press Conference: Chronic Fatigue Syndrome - part 2 (video) | journal = The Lancet TV | date = Feb 17, 2011 | url = https://www.youtube.com/ | |||
</references> | </references> | ||
| Line 2,991: | Line 921: | ||
[[Category:Psychological paradigm]] | [[Category:Psychological paradigm]] | ||
[[Category:History]] | [[Category:History]] | ||
[[Category:Seminal works]] | |||
Latest revision as of 14:11, July 25, 2023
This article needs cleanup to meet MEpedia's guidelines. The reason given is: Too long. Please consider splitting content into other articles, or condensing it (2022) |
The PACE Trial study (short for "Pacing, graded Activity, and Cognitive behaviour therapy; a randomised Evaluation") was a large and controversial trial of treatments for people with Chronic Fatigue Syndrome (CFS), also known as Myalgic Encephalomyelitis (ME).[1]
The PACE trial compared standardised specialist medical care (SMC) alone to SMC plus Adaptive Pacing Therapy (APT), Cognitive Behavioral Therapy (CBT), or Graded Exercise Therapy (GET). The researchers that conducted the trial expected that the CBT and GET groups would see the greatest improvement,[1] and this finding was reported in subsequent articles published by them.[2][3][4] This claim has been controversial, however, due to patient groups finding that exercise made patients deteriorate and CBT lacked benefit, and concerns regarding the methodology and refusal to release the full data.[5][6][7] Some patients campaigned for the full release of the PACE study's results, and after a five year battle, the PACE trial data was released for others to analyse.[8] The full PACE data led to over 40 scientists writing an open letter to the Lancet to raise serious concerns about the study, and asking for a new, independent analysis of results.[7]
The PACE trial has been highly influential in clinical guidelines in the UK, and other countries, in both government funded health care and private medical insurance, and had a significant influence on the Cochrane reviews for ME/CFS treatments.[9][10][11] By the time the main PACE trial results were published, the UK had included CBT and GET in clinical guidance for four years - the PACE trial was expected to confirm that they were effective.[9]
Background[edit | edit source]
The PACE trial[2] was funded by the UK Medical Research Council, Department of Health and Social Care (UK) for England, Scottish Chief Scientist Office, and - apparently uniquely for a clinical trial - the Department for Work and Pensions - the government department for sickness, disability and pension benefits. The PACE trial cost £5 million[12] and is the most expensive piece of research into ME/CFS ever conducted.
Recruitment of patients began in March 2005 and data collection was completed in January 2010.[2] The study protocol was published in BMC Neurology in 2007.[13] The main study outcomes were published in The Lancet in 2011[2] and the experimenters continued to publish papers on the PACE trial for a number of years.
The principal investigators were Professors Peter White, Trudie Chalder, and Michael Sharpe. Although not an author, Professor Simon Wessely provided feedback on their report.[2] He stated in November 2015 that "there are also more trials in the pipeline".[14]
Study design[edit | edit source]
641 patients were randomised into four groups in the study.[2] All received specialist medical care (SMC), which consisted of medication for symptoms such as insomnia and pain, and general advice to avoid extremes of rest and inactivity.[15] One group received SMC alone. Patients in another group additionally received adaptive pacing therapy (APT), and were advised to stay within the limits of activity imposed by the disease to give their bodies the best chance of recovery.[16] The other two groups were both told that they were not ill but deconditioned, and that if they gradually increased their activity, there was nothing to prevent their recovery.[17][18] The cognitive behavioural therapy (CBT) group focused on addressing their presumed fear of activity while the graded exercise therapy (GET) group focused on increasing their activity in a structured manner, with regular aerobic exercise as the eventual goal.
Patients in the APT, CBT and GET groups were offered up to 14 sessions with a therapist over a six-month period, to support them in following their therapy programmes, with a top-up session at 36 weeks. They also received a lengthy manual[16][17][18] explaining their therapy. All participants were offered at least three sessions of SMC.
Patients were assessed at baseline, 12 weeks, 24 weeks and 52 weeks. The main outcome measures were self-rated fatigue and physical function. Secondary measures included the study's objective variables such as a six-minute walking test, a fitness test and economic measures including the number of days of work lost due to fatigue, and the receipt of sickness benefits.[13]
Patients were also followed up (using subjective ratings only) at least two years after randomisation.[4]
As of May 7, 2018, the PACE manuals were not retrievable from the QMUL website, but they were retrievable as of 16 July 2022.[19]
All documents pertaining to the PACE trial can be found following the link PACE trial documents.
Findings[edit | edit source]
The trial's results showed that patients in the CBT and GET groups improved more in self-rated fatigue and physical function than the APT or SMC-only groups.[2] Apart from the GET group improving slightly more than the others on the six-minute walking test,[2] all of the study's objective measures[20][21] and the long-term follow-up data[4] (self-ratings of fatigue and physical function) showed no difference between groups.
The authors reported, in a 2013 paper specifically about recovery, that 22% of patients in the CBT and GET groups had recovered following these therapies, compared to 8% in the APT group and 7% in the SMC-only group.
Impact[edit | edit source]
The PACE trial and other studies that use the Oxford criteria for diagnosis of ME/CFS have had a major international impact on popular perceptions of the disease and on public policies on treating and researching it.
Media[edit | edit source]
On February 27, 2011, when the first PACE trial paper was published, researchers Michael Sharpe and Trudie Chalder held a press conference[22][23][24] to discuss their findings. Chalder stated, "twice as many people on graded exercise therapy and cognitive behaviour therapy got back to normal."[25] That assertion has been criticized for grossly overstating the study's actual findings.[26][27][28]
The claims made about the study were covered in the UK and international press.[29][30][31][32][33] For example, The Daily Mail stated, "Fatigued patients who go out and exercise have best hope of recovery",[34] while The New York Times declared "Psychotherapy Eases Chronic Fatigue Syndrome".[35] According to the British Medical Journal's report on the trial, some participants were "cured."[26]
Many other PACE papers followed, although with relatively little media attention until October 2015, when long-term follow-up results were published in The Lancet Psychiatry.[4][36][37][38][39][40][41] The Daily Telegraph ran a front-page story with the headline, "Exercise and positivity can overcome ME."[42][43] The piece stated, "Chronic Fatigue Syndrome is not actually a chronic illness and sufferers can overcome symptoms by increasing exercise and thinking positively, Oxford University has found". The article quoted Professor Sharpe describing ME as a "self-fulfilling prophesy" that happens when patients live within their limits. The article was altered following public pressure but no formal retraction was made. Science Magazine also published an article in October 2015 along with comments from Sharpe about the growing criticism outwith the patient community from the broader science community.[44]
In the UK the Science Media Centre is a government-funded body that describes its purpose as being to improve science journalism. Its reporting on ME/CFS has been criticized for bias towards a psychological etiology for the disease.[45]
Influence on treatment[edit | edit source]
The large size of the PACE trial has meant that it has had a substantial impact on the evidence base in ME/CFS. Together with other studies of CBT and GET, it is highly influential in UK clinical policy and that of many other countries, both in terms of healthcare provided by government[9] and by private medical insurance.[10][11] The influential Cochrane exercise therapy review relied significantly on the PACE trial.[46]
In the UK, the NICE guidelines for National Health Service (NHS) provided care[9] recommend CBT and GET for ME/CFS. They were published in 2007, before the PACE trial was conducted, but the evidence was based on a few small trials and was considered "somewhat limited".[47] The ME Association has asked for the guidelines to be updated to take into account new treatment evidence, noting, "we assume that the guideline surveillance review that took place in March 2011, and which followed publication of the PACE trial in February 2011, simply ‘rubber stamped’ the 2007 NICE guideline recommendations on the basis that the PACE trial had supported the recommendations relating to CBT and GET."[48] NICE responded, "we still do not feel that the evidence base is substantially evolving in this area at this time".[49] After considerable objections, this decision was reversed and the NICE guidelines clinical review began in 2017.[50]
In the US, the Mayo Clinic, Cleveland Clinic, Kaiser Permanente, and numerous key secondary medical education providers, such as UpToDate and WebMD, recommend CBT and GET, using PACE as a reference. CBT and GET were included in the Center for Disease Control's clinical guidelines for CFS, based on the PACE trial evidence.[51]
Criticisms of the study[edit | edit source]
Selection of patients[edit | edit source]
The PACE trial used the Oxford criteria for diagnosis. Many patients and specialist clinicians consider them overly broad,[52][53] and the National Institutes of Health 2015 P2P report[54] on ME/CFS recommended that the Oxford definition be retired for this reason.
Changes in criteria for effectiveness and recovery[edit | edit source]
The authors abandoned their protocol-specified main outcome and recovery analyses partway through the trial and replaced them with others.[2][3] They have defended the changes, noting, "All these changes were made before any outcome data were analyzed (i.e. they were pre-specified), and were all approved by the independent PACE Trial Steering Committee and Data Monitoring and Ethics Committee."[55] However, 42 scientists, in an open letter to the Lancet, stated that the changes were of "of particular concern in an unblinded trial like PACE, in which outcome trends are often apparent long before outcome data are seen. The investigators provided no sensitivity analyses to assess the impact of the changes and have refused requests to provide the results per the methods outlined in their protocol."[56]
Most notably, the authors introduced post-hoc "normal ranges" for fatigue and physical function.[2] These ranges have been heavily criticised for having thresholds so low that patients could worsen from trial entry and yet be within these normal ranges. The "normal range" for physical function (measured on the SF-36 100-point scale) was 60 and above, even though patients had to score 65 or lower to enter the trial. A score of 60 is close to the mean physical function score (57) of patients with Class II coronary heart failure.[57]
"The average age of participants in the PACE trial is about 39 years old; normative data suggest that people in this age group should have SF-36 scores of about 93. Yet the new 2013 “normal” is a score of 60."[58]
The PACE authors used the "normal ranges", in conjunction with other thresholds, to define clinical effectiveness in the Lancet[59] paper and recovery rates in a later paper in the Journal of Psychological Medicine.[60]
All Freedom of Information requests to the authors for the main outcome and recovery results according to the protocol-specified analyses, or for the underlying data so that others could conduct the analyses, have been refused.[61][62][63][64]
Use of subjective main outcome measures[edit | edit source]
The study has been criticised for having subjective primary analyses in an unblinded trial.[65] Subjective measures are known to be susceptible to bias, as can arise from expectations and social pressure. The CBT and GET groups, but not the others, were told that there was nothing to stop them from recovering if they gradually increased their activity, and critics have argued that these differential expectations could have inflated their self-assessments.[7][66]
Conflicts of interest and lack of informed consent[edit | edit source]
The forty-two scientists and clinicians who wrote an open letter to the Lancet complaining about the PACE trial criticized the study authors' failure to disclose a potential conflict of interest to trial participants.[56] They wrote:
"The investigators violated their promise in the PACE protocol to adhere to the Declaration of Helsinki, which mandates that prospective participants be 'adequately informed' about researchers’ “possible conflicts of interest.” The main investigators have had financial and consulting relationships with disability insurance companies, advising them that rehabilitative therapies like those tested in PACE could help ME/CFS claimants get off benefits and back to work. They disclosed these insurance industry links in The Lancet but did not inform trial participants, contrary to their protocol commitment. This serious ethical breach raises concerns about whether the consent obtained from the 641 trial participants is legitimate."
Newsletter to participants[edit | edit source]
The investigators published newsletters for participants[67][68][69][70] while the trial was still underway. Critics have said that the material in the third newsletter[69] could have influenced patients' self-reported outcomes. It included a number of positive testimonials from patients in the trial, but without naming their therapies. The PACE authors have argued that this meant that there would be no bias in favour of CBT and GET[55] but Professor James Coyne has dismissed the idea that bias would be expected to affect all four groups equally.[71]
The newsletter did, however, announce that the new NICE guidelines, "based on the best available evidence... recommended therapies [that] include Cognitive Behavioural Therapy, Graded Exercise Therapy and Activity Management." There was no explanation of what "Activity Management" was: no group had that title in the PACE trial. Dr. Bruce Levin, a professor of biostatistics at Columbia University and an expert in clinical trial design, said, "To let participants know that interventions have been selected by a government committee 'based on the best available evidence' strikes me as the height of clinical trial amateurism".[26] The newsletter also contained a less than positive assessment of research on the possibility of an infectious component of ME/CFS, including research by Jose Montoya on herpesviruses and by John Chia on enteroviruses. The newsletter said of Dr Chia's work, for example, "The laboratory work looked convincing, but many patients had significant gastro-intestinal symptoms and even signs, casting some doubt on the diagnoses of CFS being the correct or sole diagnosis in these patients." It is possible that this negative view of evidence of an ongoing infection would have made the rationale for APT appear less plausible and that for CBT and GET more plausible, thus biasing the participants.
An account of another study, in contrast, gave a positive assessment of CBT, saying "cognitive behaviour therapy was associated with an increase in grey matter of the brain and this increase was associated with improved cognitive function".
Risks and side effects[edit | edit source]
A survey[6] conducted by the ME Association in 2012 showed that 74% of patients had their symptoms worsen after a course of GET. In contrast, the PACE trial found no apparently meaningful difference in rates of adverse events between the four trial groups,[72] suggesting that APT, CBT and GET added no risk to SMC alone (since all four groups received SMC). However, critics have questioned whether patients actually increased their activity sufficiently in the CBT and GET groups to trigger many serious adverse events:[73][74] the lack of improvement in the step-fitness test in all groups indicates that this is distinctly possible.[21]
Analysis by "citizen-scientists" - ME Sufferers and Charities[edit | edit source]
Numerous ME sufferers and other interested parties have produced critiques of the PACE trial including statistical analyses and trial methodology.
Mr Courtney has written a number of published letters in the medical journals, criticising PACE.
"Chalder and colleagues acknowledge that the trial outcomes do not support the hypothetical deconditioning model of GET for chronic fatigue syndrome".[75]
Peter Kemp has written a detailed thirty page critique of the study split into ten sections called the = h.d4e0wlbznjk1 'PACE Trial Analysis'.[76]
Angela Kennedy has made specific critiques of PACE regarding the following areas:[77]
- Serious risks to clinical patient safety caused by unsound claims made about the efficacy of CBT and GET following the PACE trial;
- Gross discrepancies between research and clinical cohorts, and how clinical patients (and the physiological dysfunction associated with them) appear to have been actively excluded from PACE and other research by the research group involved in PACE, which has, ironically, caused serious resulting risks to clinical patient safety in the UK in particular;
- Related to the above, gross discrepancies in how various sets of patient criteria were used (and/or rejected), including but not limited to a changing of the London criteria by PACE authors from its original state, a set of criteria which was already controversial and problematic to start with for a number of reasons;
- Failure of the PACE trial authors to acknowledge the range and depth of scientific literature documenting serious physiological dysfunction in patients given diagnoses of ME or CFS, and how CBT and GET approaches may endanger patients in this context;
- The inclusion of major mental illnesses in the research cohort;
- The distortion by PACE trial researchers of 'pacing' from an autonomous flexible management strategy for patients into a therapist led Graded Activity approach;
- The post hoc dismissal of adverse outcomes as irrelevant to the trial, in direct contradiction to what is scientifically known about the physiological dysfunctions of people given diagnoses of Myalgic Encephalomyelitis or Chronic Fatigue Syndrome;
- The instability of 'specialist medical care' as a treatment category, and the lack of any sound category of 'control' group.
Dutch patient Frank Twisk of the ME-de-patiënten Foundation has also published criticism of the PACE trial.[78]
"The PACE trial investigated the effects of CBT and GET in chronic fatigue, as defined by the Oxford criteria, not in chronic fatigue syndrome, let alone myalgic encephalomyelitis".
"[T]he positive effect of CBT and GET in subjective measures, fatigue and physical functioning, cannot be qualified as sufficient. Mean short form-36 physical functioning scores in the CBT group (62·2) and the GET group (59·8) at follow-up were below the inclusion cutoff score for the PACE trial (≤65)3 and far below the objective for recovery as defined in the PACE protocol (≥85)."
"The vast majority of patients improved subjectively by specialist medical care and APT to the same level as by CBT and GET, without any additional therapies, including CBT and GET, or by other therapies."
"[L]ooking at subjective outcomes at follow-up and objective outcomes in earlier studies, such as physical fitness, return to employment, social welfare benefits, and health-care usage, CBT and GET, like specialist medical care and APT, cannot be qualified as effective".
Tom Kindlon a patient and Vice-Chairman of the Irish ME/CFS Association. He has published extensive criticism of the PACE trial. In 2011 he published a paper on harms associated with Graded exercise therapy.[73]
Mr Kindlon has also written a large number of letters and comments that have been published in medical journals in response to the published papers.
Among many other published letters that have been critical of the trial, many are from people who have identified themselves as patients. For example, most of the letters published by The Lancet criticising the 2011 PACE paper were from patients or representatives of patients' groups.[79][80][81]
ME Analysis: Evaluating the results of the PACE Trial study
Janelle Wiley and Graham McPhee also collaborated with others from Phoenix Rising to create 'ME Analysis: Evaluating the results of the PACE Trial' website summarising their critique of the trial.
It examined the flaws with the PACE trial and came up with 10 conclusions. The 'ME Analysis: Evaluating the results of the PACE Trial' is presented in the form of 10 conclusions. Clicking on each conclusion will lead to a short summary, and clicking on the links underneath each summary will, if you wish, lead you ever deeper into our analysis.
ME Analysis videos by Phoenix Rising
Members of Phoenix Rising including Graham McPhee and Tom Kindlon have collaborated on a set of explanatory videos about flaws in PACE's statistical analyses on YouTube:
- Video 1: The PACE Race
- Video 2: 60 - The new 75
- Video 3: Not so bad
- Video 4: The force of LOGic
- Video 6: ME Recovery Song
- Video 7: How's That Recovery?
ME Charities criticism and complaints
Charities have criticised it including Invest in ME Research.[82] Jane Colby of Tymes Trust wrote a letter to the Guardian.[83] The ME Association have also criticised the PACE trial.
MEAction
MEAction submitted a petition to retract the PACE trial that received over 12,000 signatures.[84] In addition, #MEAction published a 'PACE Trial overview' of the PACE trial and its flaws, produced by patients.[85] MEAction have also produced a factsheet of 'Why ME patients are critical of the PACE trial' which addresses three major myths which have been created by the PACE trial investigators and the harms that these myths have caused. The myths busted included:
MYTH: 1 'The controversy is fueled by a vocal minority of "vociferous" ME militants on the internet,
MYTH 2: M E sufferers oppose GET because they are afraid of exercise.
MYTH 3: ME sufferers oppose CBT because they are afraid of the stigma of mental illness.[86]
Briefing document from a Science for ME working group
Consisting of patients including Tom Kindlon, Sean Kirby and Graham McPhee, this working group has produced a document to concisely explain the flaws in the PACE trial.
ME Analysis videos (2018)
Graham McPhee has created a set of three videos looking again at the PACE trial.
Others including Jessica Kellgren-Fozard, a vlogger and TV producer with a large social media following, created a video in May 2018 called 'Have you been misled...? // What is PACE? // Medical Scandal' which had been viewed over 35,000 times.[87]
Controversy[edit | edit source]
The PACE Trial has been heavily criticised by patient groups and some researchers and science journalists for a number of methodological problems since its publication. [26][27][28][55][88][89][90][91][92]
Prof Malcolm Hooper's complaints[edit | edit source]
Prof Malcolm Hooper and Margaret Williams have followed the PACE trial from its inception in 2004 and provided salutary warnings about the possible issues and problems with the conduct of the trial due to their previous knowledge of the principal investigators research.[93][94]
They then published a 400 page critique of the PACE trial in February 2010 'Magical Medicine: How to make a disease disappear'.[95] In February 2011 upon publication of the PACE trial findings, Prof Hooper submitted a comprehensive complaint to the editor of the Lancet[96] and a further detailed response.[97]
Prof Hooper et al in 2011 published further concerns about the PACE trial[98] and have also examined the role of the Science Media Centre and the insurance industry with the PACE trial.[99] The Key Concerns about the PACE trial were also published in 2013 by MEActionUK.[100] Prof Hooper has published a summary of the key dates and chronology of the trial since 2004.[101]
Investigation by public health journalist and academic, Dr. David Tuller[edit | edit source]
Renewed interest in the trial came in October 2015 with public health expert and investigative journalist Dr. David Tuller's investigative "Trial by Error: The Troubling Case of the PACE Chronic Fatigue Syndrome Study" publication on Virology Blog which gave a detailed analysis of PACE's methodological problems. Dr. Tuller continues to publish articles criticizing different aspects of the trial.[102] Some of the main articles for the "Trial by Error" series on the PACE trial are listed below:
- Oct 21, 2015 "Trial by Error: The Troubling Case of the PACE Chronic Fatigue Syndrome Study"
- Oct 22, 2015 "Trial by Error: The Troubling Case of the PACE Chronic Fatigue Syndrome Study (second instalment)"
- Oct 23, 2015 "Trial by Error: The Troubling Case of the PACE Chronic Fatigue Syndrome Study (final installment)"
- Nov 13, 2015 "An open letter to Dr. Richard Horton and The Lancet"
- Jan 7, 2016 Trial By Error, Continued: Did the PACE Trial Really Prove that Graded Exercise Is Safe? (with Julie Rehmeyer)
For a longer list of the main articles on the PACE trial see:
The PACE trial authors, with the exception of their response to Virology on October 30, 2015, have refused to respond to or engage with David Tuller about these concerns; Tuller made requests for comment in 2015 and 2016. Similarly, Richard Horton, editor of the Lancet has refused to respond to Tuller about these concerns or about the retraction of the PACE trial publication. Sir Simon Wessely on behalf of the PACE trial principal investigators did publish an article in November 2015 The PACE trial for chronic fatigue syndrome: choppy seas but a prosperous voyage referring to the growing concerns over the PACE trial. This was published in a blog website called National Elf Service run by the Mental Elf, in which he used an analogy of the clinical trial as an ocean liner crossing the Atlantic.[14]
Further criticism from scientific community[edit | edit source]
Dr James Coyne, Professor of Health Psychology, at the University Medical Centre Gronigen]] (UMCG), published on PLoS One Blog on 29 October 2015 "Uninterpretable: Fatal flaws in PACE Chronic Fatigue Syndrome follow-up study".[71] He gave a talk at Edinburgh University in November 2015 criticising the PACE trial.[103][104][105][106] He spoke again about the PACE study in Belfast in February 2016 where he described it as "a wasteful trainwreck of a study".[107][108] Professor Coyne has also questioned whether the PACE trial paper could ever have been properly peer-reviewed, given the large number of study authors and the small world of British science.[109] He has continued to critique the PACE trial.[110]
Dr Keith Laws, Professor of Neuropsychology at the University of Hertfordshire has also criticised the PACE trial in a number of blogs on his website in November 2015.[111][112] He also co-authored a letter in the Lancet Psychiatry in 2016 with Dr. Coyne "Results of the PACE follow-up study are uninterpretable".[113]
Science journalist Julie Rehmeyer published an article in Slate magazine in November 2015 called "Hope for Chronic Fatigue Syndrome: The debate over this mysterious disease is suddenly shifting" and contrasted the hope that sufferers had with research being conducted in the US and the UK researchers involved with PACE trial.[114] Julie Rehmeyer has continued to criticise the PACE trial with further publications and in conferences.[115][116][115]
Lucy Bailey, a former editor of the Lancet, analysed the PACE trial and provided her comments on the controversy, concluding, "Psychiatrists need to understand that their presence anywhere near this condition is now toxic, and maybe they need to take a step back".[117] Bailey also wrote a letter to the Lancet Infectious Disease called "A case for Retraction?" and has questioned the reasons why actigraphy was dropped late in the trial.[118][119]
Dr Mark Vink, a family physician in the Netherlands, published in the Journal of Neurology and Neurobiology in March 2016 that "[t]he PACE Trial Invalidates the Use of Cognitive Behavioral and Graded Exercise Therapy in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome".[120]
Prof Jonathan Edwards has declared that "PACE is valueless for one reason: the combination of lack of blinding of treatments and choice of subjective primary endpoint. Neither of these alone need be a fatal design flaw but the combination is". He also stated "the authors have not been meticulous in trying to avoid bias that might arise. On the contrary they appear to have acted in ways more or less guaranteed to maximise bias."[66]
In early 2016, Leonid Schneider, a science journalist, criticised the lack of data sharing by the PACE trial authors and the Lancet and its editor Richard Horton's role in the scandal and compared it to other recent Lancet scandals.[121][122][123]
Prof Andrew Gelman, Professor of Statistics and Political Science at Columbia University, NY, examined the Lancet's role in how the PACE trial got taken so seriously and stayed afloat for so long.[124][125][126][127]
Dr Sten Helmfrid published an article in the journal Socialmedicinsk tidskrift in September 2016 called "Studies on Cognitive Behavioral Therapy and Graded Exercise Therapy for ME/CFS are misleading" that criticized not only the PACE trial, but the entire CBT/GET paradigm for ME/CFS. "The underlying model has no theoretical foundation and is at odds with physiological findings. Surveys suggest that the efficacy of CBT is no better than placebo and that GET is harmful. Therefore, cognitive behavioral therapy and graded exercise therapy for ME/CFS are not evidence based."[128]
Dr Sonia Lee, a clinical epidemiologist, published 30 slides critically appraising the PACE trial in February 2017 and concluding the PACE trial authors should "rectify or retract".[129][130] Dr Lee self-published Research Waste In ME/CFS which compared psychosocial trials using the PACE trial as an example psychosocial trial. Lee found that psychosocial trials were more likely to engage in selective reporting than biomedical trials, that PACE in particular had significant design, evidence and justification weaknesses or omissions, and concluded "it confirms the concerns raised by ME/CFS groups that psychosocial interventions are harmful, and present questionable therapeutic benefits no different to a placebo".[131]
Professor Leonard Jason in the Journal of Health Psychology wrote in February 2017 'The PACE trial missteps on pacing and patient selection' which criticised the issue of patient selection in the trial with the use of the Oxford criteria included those without the disease and the PACE trial investigators did not design and implement a valid pacing intervention in the trial.[132]
Dr Sarah Myhill in her August 2017 video on Medical Abuse in ME Sufferers (MAIMES) called the PACE trial "fraudulent" and "a criminal act" and is an "abuse" of "vulnerable people" and "defrauds them of benefits".[133]
In April 2018, ME/CFS researcher Dr Neil McGregor sent his analysis of the harms in the PACE studies, to Australia's Chief Medical Officer. In it, McGregor stated that "The conclusions within the PACE studies is consistent with a highly biased study which under-estimates, and does not assess, the adverse outcomes in relationship to the disease process. The use of therapies of this type cannot be recommended based upon the data within the PACE study and must exclude the worst affected patients as they were not included in the study assessments."[134][135]
Scientists' open letter to The Lancet and Psychological Medicine[edit | edit source]
In November 2015, scientists Ronald Davis, David Tuller, Vincent Racaniello, Jonathan Edwards, Leonard Jason, Bruce Levin and Arthur Reingold wrote an open letter to The Lancet citing "major flaws" in the original trial publication and asking for an independent re-analysis of the individual-level trial data.[7] The journal failed to respond.
In February 2016 the open letter was re-published with 36 additional signatures from doctors and researchers including: Dharam Ablashi, Lisa Barcellos, James Baraniuk, Lucinda Bateman, David Bell, Alison Bested, Gordon Broderick, John Chia, Lily Chu, Derek Enlander, Mary Ann Fletcher, Kenneth Friedman, David Kaufman, Nancy Klimas, Charles Lapp, Susan Levine, Alan Light, Sonya Marshall-Gradisnik, Peter Medveczky, Zaher Nahle, James Oleske, Richard Podell, Charles Shepherd, Christopher Snell, Nigel Speight, Donald Staines, Philip Stark, John Swartzberg, Ronald Tompkins, Rosemary Underhill, Rosamund Vallings, Michael VanElzakker, William Weir, Marcie Zinn and Mark Zinn.[56]
Richard Horton, the editor of the Lancet, requested it to be submitted as an official letter but after 6 months of chasing he rejected it and refused to publish the letter.[136][137]
On March 13, 2017 David Tuller and the original signatories to the open letter to the Lancet in 2016 and an additional 37 signatories signed an open letter to the Journal of Psychological Medicine. In total 102 signatories signed this open letter regarding the recovery paper of 2013 to the Journal of Psychological Medicine.
These included scientists and medical professionals including Molly Brown, Todd E. Davenport, Simon Duffy, Meredyth Evans, Robert F. Garry, Keith Geraghty, Ian Gibson, Rebecca Goldin, Ellen Goudsmit, Maureen Hanson, Malcolm Hooper, Betsy Keller, Andreas M. Kogelnik, Eliana M. Lacerda, Vincent C. Lombardi, Alex Lubet, Steven Lubet, Patrick E. McKnight, Jose G. Montoya, Henrik Nielsen, Elisa Oltra, Nicole Porter, Anders Rosén, Peter C. Rowe, William Satariano, Ola Didrik Saugstad, Eleanor Stein, Staci Stevens, Julian Stewart, Leonie Sugarman, Mark VanNess, Mark Vink, Frans Visser, Tony Ward, John Whiting, Carolyn Wilshire and Michael Zeineh.[138][139] Twenty-seven ME charities from seventeen countries also co-signed the open letter including European ME Alliance, 25 Percent ME Group (UK group for severe ME patients), Emerge Australia, Irish ME Trust, ME Association (UK), and International Solve ME/CFS Initiative (US).[140]
On March 23, 2017, David Tuller reported that one of the editors of the Journal of Psychological Medicine, Sir Robin Murray, responded with "an unacceptable response." Tuller restated that "That the editors of Psychological Medicine do not grasp that it is impossible to be “disabled” and “recovered” simultaneously on an outcome measure is astonishing and deeply troubling." The open letter was reposted with signatures from an additional 17 clinicians, scientists or advocates and 23 more charities including Norman E. Booth, Joan Crawford, Valerie Eliot Smith, Susan Levine, and Sarah Myhill.
An additional 25 patient charities to sign included Associated New Zealand ME Society, Deutsche Gesellschaft für ME/CFS and Lost Voices Stiftung (Germany), ME Research UK, ME/CFS (Australia) Ltd, The MEAction Network, Millions Missing Canada, National CFIDS Foundation, Inc. (US), and Open Medicine Foundation (US). No response from the Lancet was received - campaigning in 2018 continued.
Petitions and Protests[edit | edit source]
On October 28, 2015, The MEAction Network launched a petition addressed to The Lancet, Psychological Medicine and the PACE trial authors, calling for an independent analysis of the data and the retraction of some of the PACE trial's "misleading claims" based on "absurd 'normal ranges' for fatigue and physical function". The petition was closed in February 2016, having gathered 11,897 signatures from people in sixty-four countries.[5][141]
A US petition to the Agency for Healthcare Research and Quality (AHRQ) and the Centers for Disease Control was also launched in late 2015, asking government agencies to remove guidelines and recommendations based on PACE and other studies using the Oxford definition of ME/CFS.[142]
On International ME Awareness Day (May 12th) in 2017 due to the intransigence of the PACE researchers for years, a Millions Missing protest in London resulted in hundreds of patients protesting against the PACE trial.[143]
Breaches of patient's data security by study investigators[edit | edit source]
In 2006 confidential PACE trial patient data was stolen from an unlocked drawer at King's College London.[144]
Malcolm Hooper documents in Magical Medicine how in 2005 confidential patient data was erroneously released by PACE author Professor Michael Sharpe in relation to another study.[95]
The Centre for Welfare Reform - 'In the Expectation of Recovery'[edit | edit source]
The Centre for Welfare Reform published a 64 page report in April 2016 examining the PACE trial and relating the study to the biopsychosocial model and its links and influence from the insurance industry and government welfare reforms. The report titled 'In the Expectation of Recovery' by George Faulkner heavily criticised the PACE trial and stated "The way in which the biopsychosocial model has been used and promoted, without good supporting evidence for many of the claims being made, is unethical.” and “Had homeopaths or a pharmaceutical company conducted a trial and presented results in the manner of the PACE trial the British research community would have been unlikely to overlook its problems."[145] Dr Simon Duffy writing in the Huffington Post questioned the motive of the research in 'The Misleading Research at the Heart of Disability Cuts'.[146]
Sense About Science USA[edit | edit source]
Sense about Science USA (SAS USA) published a major statistical examination of the PACE trial in March 2016. The Executive Director of SAS USA, Trevor Butterworth wrote an accompanying editorial on PACE.[147] Prof Rebecca Goldin of Mathematical Sciences at George Mason University and Director of STATS (a collaboration between SAS USA and the American Statistical Association) wrote the 7000 word statistical critique PACE: The research that sparked a patient rebellion and challenged medicine.[58]
Other major investigations and reports[edit | edit source]
In August 2016 Julie Rehmeyer presented a critique of the PACE trial at the largest gathering of statisticians in North America, the Joint Statistical Meeting 2016, in Chicago titled Bad Statistics, Bad Reporting, Bad Impact on Patients: The Story of the PACE Trial. Rehmeyer said "When I went through the slides showing the changes to the physical function criterion for recovery, I saw jaws drop."[148]
PACE Trial in UK Parliament[edit | edit source]
The PACE Trial has been the subject of a number of parliamentary enquiries by parliamentarians mainly the Countess of Mar. The Countess of Mar asked questions of the government via a short debate in the House of Lords on the assessment of the PACE trial on 6 February 2013. A video and transcript is available on YouTube and Hansard.[149][150] Comments and analysis on the PACE trial and its establishment defenders has also been made by advocates.[151]
During the court case in 2015 by Matthees and the Information Commissioners Office v QMUL it was stated by Peter White that he regarded parliamentary debates as "harassment" and had to brief those taking part in the debate.
In November 2016, Kelvin Hopkins MP asked seven written questions relating to the PACE Trial including "request that the Medical Research Council conducts an inquiry into the management of the PACE trial to ascertain whether any fraudulent activity has occurred." and "prevent the PACE trial researchers from being given further public research funding until an inquiry into possible fraudulent activity into the PACE trial has been conducted."[152]
The Countess of Mar has also asked in February 2017 the UK parliament's Committee of Public Accounts to investigate the use of public funds on the PACE Trial and the PACE authors use of public funds to resist requests to release anonymised clinical trial data. Her letter included a report which stated the trial was "professional misconduct and/or fraud" and "Professor White obtained ethical approval for the study under false pretences".[153]
Response to criticism[edit | edit source]
Response[edit | edit source]
The trial investigators have replied to some criticism of the trial but have been much criticised as being evasive and not responding to the issues raised.
Some of their response to letters to The Lancet concerning their main analyses,[154] Psychological Medicine concerning their recovery analyses,[138] and Lancet Psychiatry concerning their secondary mediation analyses [155] and long-term follow-up paper.[156] A letter to the BMJ by Tom Kindlon[157] drew a reply from the authors[158] that in turn received 31 responses of its own.[159]
Since the controversy with criticism from the scientific community they have largely not responded to the key concerns and have repeated their earlier arguments.
The investigators have also responded to a 14,000-word critique by public health expert and journalist Dr David Tuller.[55] Dr Tuller wrote a rebuttal to their response.[88] He has said, “The PACE authors have long demonstrated great facility in evading questions they don’t want to answer”.
Professor James Coyne reported that he agreed to debate the authors on health website National Elf about the trial but that they declined.[160] Professor Simon Wessely was given the vacated National Elf spot and wrote a lengthy article praising the trial, noting that he once described it as "a thing of beauty" and saying, "We can accept that PACE was a good trial and we can have some confidence in its findings".[14]
Allegations of harassment, death threats and smear campaign[edit | edit source]
During the criticism of the PACE trial by patient groups the PACE trial psychiatrists publicised that they were receiving death threats and harassment.
The PACE trial investigators and Professor Simon Wessely have publicly claimed they have been harassed and subjected to death threats.[161][162][163][164][165][166][167][168] A feature article in the BMJ (read by most UK doctors) was published in June 2011 called 'Dangers of research into chronic fatigue syndrome'.[169] The Times article was titled 'Doctor’s hate mail is sent by the people he tried to cure'.[170] An article was also published in the Sunday Times magazine with Simon Wessely repeating the death threats narrative.[171] One article was even called ‘It’s safer to insult the Prophet Mohammed than to contradict the armed wing of the ME brigade’.[172]
However, the PACE authors and their supporters have been accused of blurring the line between harassment and legitimate criticism of the study. Documentation obtained under the Freedom of Information Act from meetings in 2013 that were attended by some of PACE’s principal investigators include a statement that “harassment is most damaging in the form of vexatious FOIs [Freedom of Information requests].”[173] This framing of FOIA requests as harassment is widely taken to be a reference to the PACE authors, who have complained about the number of FOI requests that they have received for data[174] and who have dismissed several as "vexatious":[175][176][177] the Information Commissioner's Office was told that Professor Peter White "believes that the requests are clearly part of a campaign to discredit the trial” and that “the effect of these requests has been that the team involved in the PACE trial, and in particular the professor involved, now feel harassed and believe that the requests are vexatious in nature."[174]
The criticisms of the trial's methodology and analyses by patients and others has been referred to by the investigators - and The Lancet - as part of a campaign to undermine the the study. In an editorial comment that accompanied letters criticising the trial, The Lancet described the trial as “rigorously conducted” and questioned whether the “coordination of the response... has been born... from an active campaign to discredit the research”.[178] In an interview on Australian national radio shortly after publication, Dr Richard Horton, The Lancet’s editor, described patients who criticised the trial as “a fairly small, but highly organised, very vocal and very damaging group of individuals”.[179]
But some accuse the investigators of a campaign against patients, labelling them as harassers to undermine their criticisms of the trial, including Angela Kennedy.[180]
The PACE trial authors refused to provide anonymised data to many individuals and also refused to accept the Information Commissioners Office decision for QMUL to release the data in 2015 (see Release of Data/Information Tribunal below). During the PACE trial authors appeal to the Tribunal an article was published during this period by their associates in Nature in which they bizarrely appeared to use projection in describing disabled ME sufferers as “hard-line opponents” of research into chronic fatigue syndrome and compared them with industry lobbyists such as tobacco and climate change denialists.[181][182] Public health expert and journalist David Tuller, who has said, "Wrapping themselves in victimhood, the [PACE authors] have even managed to extend their definition of harassment to include any questioning of their science and the filing of requests for data — a tactic that has shielded their work from legitimate and much-needed scrutiny."[183][184]
The Science Media Centre (SMC) was found to have orchestrated and publicised the false narrative in 2011 in the UK media about extremists harassing researchers.[185][186] An article in the Establishment in May 2016, The Hidden Battle For The Rights Of Chronic Fatigue Syndrome Sufferers summarised the campaign to smear ME sufferers and how these psychiatrists after categorising ME as a psychological over two decades then were able to use institutional gaslighting when patients were question the scientific validity of the trial and to stop access to data requests from the PACE trial by framing them as harassment and abuse. Catherine Hale has written about the 'Politics of Stigma' created for ME sufferers by the PACE trial authors.[187] Peter Tatchell, a human rights advocate has supported ME sufferers for their human rights against the psychotherapies and the PACE trial and defended them from the smear campaigns by the psychiatrists similar to what he faced in his advocacy in the 1970s.[188][189][190] Ethics experts Charlotte Blease and Diane O'Leary have investigated ethics and injustice in the behavior of some researchers in the ME/CFS field and the effects of government and institutional actions on patients.
No evidence of anyone with ME/CFS in relation to these matters being charged by the police/law enforcement or convicted in the Courts with harassment and death threats has come to light, despite a number of ME advocates attempting to find evidence, including filing Freedom of Information Act requests to public bodies.[191][191][192][192][193]
Calls to release data[edit | edit source]
Open data commitments[edit | edit source]
The study authors have been criticised for failing to follow the requirements of the Medical Research Council (who provided significant funding for the trial) to release anonymised trial data.[194]
The 2012 PACE cost-effectiveness paper[20] was published in the journal PLoS One. That journal requires authors, as a condition of submitting their papers, to agree to release the anonymized trial data underlying the paper's analyses upon request. In November 2015 Professor James Coyne made a request to the PACE authors on that basis, but the authors treated his request as a Freedom of Information request and refused it.[195][196] However in March 2016 the PLoS One journal confirmed they had requested the investigators to release the trial data, as they committed to do prior to publication.[197][198]
In 2015 Peter White lobbied the UK government through his university QMUL and the Science Media Centre to restrict the Freedom of Information Act 2000 for universities especially for controversial research and cited the PACE trial and compared ME patients requesting data with "climate change science, and research into the health effects of tobacco".[199][200]
Freedom of Information requests[edit | edit source]
The PACE trial investigators have been asked for 160 pieces of separate information in 35 Freedom of Information requests since 2011.[174] They have dismissed at least three of these claims as "vexatious".[175][176][177][64][201]
A ruling by a UK government body, the Information Commissioner's Office (ICO), on October 27, 2015 ordered Queen Mary University of London (QMUL, the institutional base of the PACE trial's lead investigator Peter White) to release the trial data to a patient who had requested it, subject to appeal within 28 days.[202] Three Freedom of Information requests from 2012 and 2013 were included in the ICO's decision.[203][204][61] An appeal by QMUL was heard by the First-Tier Tribunal on 20-22 April 2016. QMUL responded to a freedom of information request confirming the cost of its legal fees for the tribunal totalled £245,745.27 (around USD 350,000).[205][206][207] The First-Tier Tribunal judgement was published on August 16, 2016, roundly dismissing the appeal by QMUL, and deciding that the PACE trial data should be released.[208][209] The university has not yet stated whether it will appeal the judgement.[210] The PACE patient consent form was also the released through a Freedom of Information request.[211]
Dr Richard Horton, editor of The Lancet, stated on April 18, 2011 in a national Australian radio interview: "The Freedom of Information requests and the legal fees that have been racked up over the years because of these vexatious claims has added another £750,000 of taxpayers’ money to the conduct of this study".[97]
Data requests from scientists[edit | edit source]
Professor James Coyne has publicly called for the trial data to be released. He has repeatedly criticized the PACE investigators for failing to abide by modern expectations concerning "open data".[212][213][214][215][216][217][218][219][220]
Professor Coyne's own request for the data was dismissed under the Freedom of Information Act by the study authors as "vexatious" and as having an "improper motive".[175]
Scientists Ronald Davis, David Tuller, Bruce Levin, and Vincent Racaniello requested the PACE trial data in December 2015.[221] Their request was rejected by the trial investigators.[222] On International ME Awareness day in May 2016 in an interview with an advocate's article called PACE-Gate, Dr Racaniello stated "I think they are going to ignore, obfuscate, and give their usual responses until we are all dead. I don’t have hope that the PACE authors, or Lancet, will respond in any meaningful way until there is more of an outcry."[223]
ME Charities and others calls for data release[edit | edit source]
A number of patient charity groups and individuals have called for the release of the PACE data, including some outside the ME/CFS community who advocate "open data" in science. These calls include:
- 2016: open letters from two patient charities, in Australia, ME/CFS Australia (SA) Inc and Emerge Australia, calling for the data to be released.[224][225]
- 2016: an open letter the European ME Alliance (EMEA) representing patient group charities from 12 European countries (Belgium, Denmark, Finland, Germany, Holland, Iceland, Ireland, Norway, Spain, Sweden, Switzerland and UK), calling for the data to be released.[140]
- 2016: an open letter by Mark Berry of Phoenix Rising, a US based non profit with the largest ME patient forum membership in the world, calling for the data to be released.[226]
- 2016: open letters from British patient charities Action for ME, Invest in ME, ME Association, Tymes Trust, ME Research UK, Hope 4 ME & Fibro NI, the Welsh Association of ME & CFS Support and the 25 Percent ME Group.[227][228][229][230][231][232][233][234][235]
- 2016: an open letter from the Irish ME Trust charity.[236]
- 2016: an open letter from a Canadian patient group, the National ME/FM Action Network.[237]
- 2016: an open letter from two Belgian patient organisations, ME-Gids and WUCB.[238]
- 2016: an open letter from a Dutch patient group, Groep ME-DenHaag / The Dutch Citizen’s Initiative for ME patients[239] and a joint letter from three further dutch patient group charities, ME/CVS Vereniging, ME/CVS Stichting Nederland, Steungroep ME en Arbeidsongeschiktheid[240]
- 2016: an article, "PACE trial and other clinical data sharing: patient privacy concerns and parasite paranoia", by Leonid Schneider, an independent science journalist.[122]
- 2015: a blog post by Dr Richard Smith, former editor of The BMJ, who said that the PACE authors' institutions were "making a mistake .. the inevitable conclusion is that they have something to hide."[241] and in article for F1000 Research.[242]
- 2016, Release the PACE trial data: My submission to the UK Tribunal, James Coyne, Aug 18, 2016.[243]
A total of 29 patient charity groups from 15 countries wrote in support of releasing the anonymized PACE trial data.[244][245]
A ME charity polled on the question "Should or should not the anonymised data from the PACE trial be released for independent analysis?" By March 17, 2016, 1391 voters took part and 99% (1378 voters) voted for 'Should be released'. 0% (5 voters) voted for 'Should not be released'.[246]
Both cofounders of Retraction Watch, Ivan Oransky and Adam Marcus, added their weight behind patients in the refusal of the PACE trial investigators to release the anonymised data in article in STAT News feature 'The Watchdogs Keeping an eye on misconduct, fraud, and scientific integrity' To keep science honest, study data must be shared and concluded "when researchers refuse to share data, and how they came up with it, they lose the right to call what they do science".[124]
This battle was reported in the Wall Street Journal by Amy Dockser Marcus on 7 March 2016 as Patients, Scientists Fight Over Research-Data Access, which featured quotes from David Tuller, Tom Kindlon, Anna Sheridan Wood, James Coyne.[247] Professor Peter White responded to the article with a letter to the WSJ published 24th of March, 2016, claiming that "The main reason that my colleagues and I have been unable to release data to members of the public who ask for it is that we don’t have the consent of the trial participants to release their data in this way, and we are ethically bound to act in the best interest of our patients."[248]
Release of Data[edit | edit source]
Alem Matthees, an Australian ME sufferer, submitted a FOIA request to the PACE trial authors who were ordered to provide the requested data by the Information Commissioners Office. This decision was appealed by them to the Information Tribunal and was heard on April 20th 2016 at a three day hearing.
Information Tribunal[edit | edit source]
Mr Alem Matthees' original PACE trial data Freedom of Information Act request for the release of PACE trial data to Queen Mary University of London (QMUL) was submitted on March 24, 2014. QMUL refused to release the requested data and Mathees complained to the Information Commissioners Office. The Information Commissioners decision was made on October 27, 2015 and concluded that QMUL should release the data.[249][202] The PACE investigators appealed on November 23, 2015.
QMUL appealed the decision, wishing to continue to withhold the PACE trial data, which led to a tribunal in 2016.[250][251] The Information Commissioner's response to the Tribunal as First Respondent of January 12, 2016 and Mr Alem Mathees Main response to Tribunal as Second Respondent was also submitted to the Tribunal for the three day hearing from April 20th-22nd, 2016.[252] The Information Rights Tribunal Judgement was finally published on August 16, 2016.[209][253] The Tribunal upheld the original ICO decision and rejected the appeal by the PACE investigators and ordered QMUL to release the anonymised data to Mr Matthees.
The tribunal took evidence under the normal rules of court. The tribunal also concluded of the expert witness for the PACE authors that "It was clear that his assessment of activist behaviour was, in our view, grossly exaggerated and the only actual evidence was that an individual at a seminar had heckled Professor Chalder" and "clearly in our view had some self-interest, exaggerated his evidence and did not seem to us to be entirely impartial. What we got from him was a considerable amount of supposition and speculation, with no actual evidence to support his assertions or counter the respondents arguments." The tribunal panel noted that the Commissioner had referred to Professor Anderson's "wild speculations" that "young men, borderline sociopathic or psychopathic" would attempt to identify trial participants from the anonymised data, and said that his views "do him no credit".[254]
The decision noted in the evidence that "Contrast instead Professor Chalder when she accepts that unpleasant things have been said to and about PACE researchers only, but that no threats have been made either to researchers or participants. The highest she could put it was some participants stated that they had been made to feel "uncomfortable" as a result of their contact with and treatment from her, not because of their participation in the trial per se. There is no evidence either of a campaign to identify participants nor even of a risk of an 'insider threat'."
Moreover, regarding the independent Cochrane review it was admitted in the tribunal that "Professor Chalder states that disclosure to the Cochrane review does not count as disclosure to independent scientists as all three of the PACE principal investigators sat on the review panel."
Additionally, a UK ME sufferer submitted a FOI request in June 2016 and established that the PACE trial investigator's university paid £245,745.27 for legal fees to defend their case in the tribunal against the original ICO decision.[255][256]
The lead PACE investigator's university issued a statement the same day stating "We are studying the decision carefully and considering our response".[210] An open letter in support of Alem Matthees was sent to the university and signed by Dr Ronald Davis, Dr Jonathan Edwards, Dr Rebecca Goldin, Dr Bruce Levin, Dr Zaher Nahle, Dr Vincent Racaniello, Dr Charles Shepherd and Dr John Swartzberg strongly urging them to not appeal further.
The ME community through #MEAction posted a plea to the PACE trial lead authors to "pursue a complete retraction of their PACE trial paper from the Lancet and all associated subsequent papers from their relevant journals." and to "[e]nd this tragedy now."[257] QMUL did not appeal and released the data to Matthees.[258] QMUL finally decided not to appeal a second time, and released the PACE trial data.[259]
Results of Reanalysis of the PACE trial data[edit | edit source]
Virology blog published the re-analysis on September 21, No 'Recovery' in PACE Trial, New Analysis Finds and concluded "The results should put to rest once and for all any question about whether the PACE trial's enormous mid-trial changes in assessment methods allowed the investigators to report better results than they otherwise would have had. While the answer was obvious from Dr. Tuller's reporting, the new analysis makes the argument incontrovertible."
The full re-analysis was published as A preliminary analysis of 'recovery' from chronic fatigue syndrome in the PACE trial using individual participant data and was conducted by Alem Matthees, Tom Kindlon, Carly Maryhew, Philip Stark and Bruce Levin and stated "This re-analysis demonstrates that the previously reported recovery rates were inflated by an average of four-fold.".[260]
On August 19, 2016 The Centre for Welfare Reform also published an update to its earlier 64 page report from April 2016.[261]
A critical commentary and preliminary re-analysis of the PACE trial was published in December 2016 in the Journal of 'Fatigue: Biomedicine, Health & Behavior' by Dr Carolyn Wilshire, Tom Kindlon, Alem Matthees and Simon McGrath which found that "The claim that patients can recover as a result of CBT and GET is not justified by the data, and is highly misleading to clinicians and patients considering these treatments.[260][262]
'A Rejoinder to Sharpe, Chalder, Johnson, Goldsmith and White' was published by Dr Carolyn Wilshire, Tom Kindlon and Simon McGrath in response to the PACE authors reply to their original publication .[263] Wilshire et al disproved again the PACE trials misleading claims as recovery was a strong claim and did not adhere to the core meaning of the word, no evidence that the original protocol specified definition was too stringent and absolute recovery rates from other studies were not legitimate source of support for the recovery definition used. It reinforced the original conclusion that "The PACE trial provides no evidence that CBT and GET can lead to recovery from CFS. The recovery claims made in the PACE trial are therefore misleading for patients and clinicians".
Instead of engaging with critics by releasing other additional requests for data, the PACE trial authors updated their guidelines in January 2017 for those requesting access to the PACE trial data which further restricted access by requiring "formal agreement" with "precise analytic plans, and plans for outputs".[264] Prof James Coyne commented that it is "a ruse to trap the unwary in endless haggling and appeal, while protecting the PACE investigators from the independent reanalysis of the claims, which they have already declared poses a reputational risk to them".[265]
Scientific and Media response[edit | edit source]
Despite patients and critics not having access to the Science Media Centre the media coverage especially outside the UK was extensive. For a full list of the scientific and media response to the PACE trial data release and reanalysis, see the article:
Journal of Health Psychology 'Special Issue on the PACE Trial'
A special edition of the Journal of Health Psychology was published on July 31, 2017, by the editor, Dr David Marks with an editorial - Special Issue on the PACE Trial and accompanying all 20 Editorials & Commentaries.[266]
A press release was put out on July 31, 2017, called The PACE Trial: The Making of a Medical Scandal.[267][268]
Prof Coyne explained in his blog of the "[l]ast ditch attempt to block publication of special issue of Journal of Health Psychology foiled" and that anonymous and powerful PACE proponents made threats to the publisher of JHP, SAGE Publications, that made them reluctant to publish the special edition. explained in his blog "Some threats were made to Sage Publications, the publisher of Journal of Health Psychology, which expressed a reluctance to go forward as planned. As often happens with these kind of pressures, we weren’t told the identity of the complainant. It was clear that whoever s/he was, this person was powerful in being able to grind to a halt of making the special issue available, complete with the introductory editorial that was not previously available.'"[269]
It transpired that "When the effort to block publication of the special issue failed, the PACE investigators got criticism posted at Science Media Centre." [270]
The Science Media Centre put out its own "expert reaction" press release to UK journalists minutes before the special edition was available to spin the story about the PACE scandal and distract and refocus away from the central problems with the scandal.[271] The Science Media Centre ignored the glaring problems and instead made personal attacks agains the authors.
Dr David Tuller deconstructed "these rather pathetic efforts at defending the indefensible" from the Medical Research Council (MRC), an unnamed University of Oxford spokesperson, and Malcolm Macleod in The Science Media Centre's Desperate Efforts to Defend PACE.[272] It was stated by a whistleblower that Michael Sharpe was the person who complained to the Journal to block publication.[273] This was published on social media by a ME advocate and although unverified, due to its importance as it was the principal author of PACE trial, it is being documented and referenced here.
The Times (UK) published two articles that covered the issue but with a spin and focus on disagreements between scientists and resignations rather than the central issues in the Special Issue on the PACE trial scandal.[274][275][276][277] The impact of the decades long denial by the PACE authors of the disability could be seen in the Express article Is chronic fatigue syndrome real? Life-threatening condition can leave sufferers bed bound.[278] The Daily Mail reported on it with Angry scientists throw insults at each other over the results of a £5 million taxpayer-funded study into chronic fatigue syndrome.[279]
US local media reported on it as 'Chronic fatigue syndrome reality conflicts with medical study'. Other international media reporting it including the Metro Netherlands 'Wetenschappers vallen door de mand'. Forskning (Research) of Norway reported it as 'Hard kritikk av stor ME-studie'/'Hard criticism of major ME study' . Seeker published on both the PACE trial scandal and the 2017 Stanford Cytokine study and concluded "The fact that this new study and the journal issue came out at virtually the same time is significant. The study and the journal "reinforce each other," Tuller said".[280]
Journalist Jerome Burns in the Daily Mail wrote the article Why are doctors and patients still at war over M.E.? How the best treatment for the debilitating condition is one of the most bitterly contested areas in medicine.[279] David Tuller commented on social media "Amazing to see a fair story on ME like this in the UK press. Seems like the PACE story has broken through" as it was a breakthrough in the UK media because of the influence of the PACE authors and the Science Media Centre.[281]
The iNews reported it as "You're a disgusting old fart neoliberal hypocrite" – scientists in furious row over ME study with a prominent and groundbreaking comment from Dr David Marks in the national UK media "The many wrongs committed by psychiatry and medicine to the ME/CFS community can only be righted when the Pace trial is ultimately seen for what it is: a disgraceful confidence trick to reduce patient compensation payments and benefits."[282]
The Medical Independent in Ireland published an article referencing Dr Keith Geraghty and Nasim Marie Jafry's work with a literary take on the changing narrative of ME from neurological to biopsychosocial and culminating in the PACE trial and a whole special issue dedicated to the scandal.[283]
The President of the IACFS/ME Prof Fred Friedberg stated of the Special Edition "the most misleading and damaging aspect of the PACE trial was contained in the subsequent reporting of "recovery"" and "This can have the effect of delegitimizing the illness even more and may discourage biomedical research on ME/CFS". He continued "misleading reports that inflated the benefits of the PACE trial, which were widely cited in the mass media at the time, may have further undermined illness credibility with the research community" as far as the United States.[284] In contrast a separate investigation into research funding it was established Peter White as receiving the most research funding and a near monopoly from the UK government and taxpayers out of all researchers despite protests from patients.[285]
Learn more
- 2018, = 11:01:20&out = 11:31:59 UK Parliament debate on ME Treatment - UK Parliament (video), Hansard transcript
- 2018, PACE trial and its effect on people with ME (alternate video #2 - UK Parliament via YouTube
- 2018, PACE trial and its effect on people with ME (alternate video #3 - UK Parliament via YouTube
- 2018, PACE trial and its effect on people with ME (alternate video #4 - UK Parliament via YouTube
- 2017, 'Special Issue on the PACE Trial' - The Journal of Health Psychology
- 2016, Bad science misled millions with chronic fatigue syndrome. Here’s how we fought back, Julie Rehmeyer, Stat News, Sep 21, 2016.[286]
Reaction by Scientific, Medical and ME Communities[edit | edit source]
The PACE trial was scientifically discredited but the impact of it on the medical community has continued especially in the UK.
Agency for Healthcare Research and Quality (AHRQ) amendment, US The US government agency called the Agency for Healthcare Research and Quality (AHRQ) revised its earlier recommendations about found that when studies of CBT and GET, including PACE, that used the Oxford criteria for CFS were excluded, there was no evidence for graded exercise and only weak evidence for CBT.[287] Mary Dimmock led the way on this issue.[287]
PLOS Expression of Concern
Prof James Coyne had requested anonymised data in 2015 from the PACE trial authors from the Cost Effectiveness paper. He pursued this request throughout 2015 and 2016 but was also refused as "vexatious". After 18 months, PLOS issued an Expression of Concern on May 2nd 2017. It stated "We conclude that the lack of resolution towards release of the dataset is not in line with the journal's editorial policy and we are thus issuing this Expression of Concern to alert readers about the concerns raised about this article.[288] The editors of PLOS One, Iratxe Puebla and Joerg Heber, wrote in PLOS One Blog "Since we feel we have exhausted the options to make the data available responsibly, and considering the questions that were raised about the validity of the article's conclusions, we have decided to post an Expression of Concern to alert readers that the data are not available in line with the journal's editorial policy".[289] Retraction Watch reported on the development.[290]
Centers for Disease Control & Prevention (CDC), US
David Tuller reported in July 2017 that the CDC had updated their treatment recommendations and that all mention of CBT and GET as treatment or management strategies had "disappeared". Tuller reported that the key members of the PACE trial had a long history with the CDC and "For advocates, the CDC's removal of the CBT/GET recommendations represents a major victory. The removal of CBT and GET has been "heralded as an important" by patient charities.[291][292]
In September, David Tuller and Julie Rehmeyer wrote an article in STAT exploring Why did it take the] CDC so long to reverse course on debunked treatments for chronic fatigue syndrome?.[293] NPR reported on the change in the CDC recommendations and that exercise can make the disease worse and criticised the seminal paper Sir Simon Wessely from 1989 that led to exercise as a treatment and the PACE trial.[294] In November 2017, the New York Times published an article stating that longstanding advice to exercise was now recognised as not only ineffective but counterproductive, and referred to the CDC and NICE decision to review the recommendations.[295]
UK reaction from the Journals - Lancet, Psychological Medicine and Guideline Provider - NICE
The UK medical and scientific establishment have been obstinate in changing their position to reflect the flaws of the PACE trial as the PACE trial investigators are highly influential and senior members of the UK establishment. The Lancet have refused to retract the study as yet. Psychological Medicine have also refused to retract the study as well.
The controversial NICE guidelines were challenged by patient groups after the PACE trial was discredited by way of petitions and challenges by ME patient groups in 2017. ME sufferers have taken other actions due to the UK medical establishment intransigence of acknowledging the issues. The Medical Research Council (MRC) and its Chief Executive continued to defend the trial and in a statement dated August 28, 2018 said that as funders of the PACE trial we reject the view that the scientific evidence provided by the trial was unsound.[296]
Jerome Burns in Health Insights UK published a follow up article to his 2016 articles on the PACE trial in the article headlined 'The claim that the cure for the crippling fatigue of ME/CFS was to change your mind always seemed bizarre. Now it really is on the way out…' The journalist stated he thought it would be big story in 2016 with lots of repercussions but the UK press largely ignored it and he revisited the story in 2019 due to professional opinions changing against CBT/GET, with the recent House of Commons debates and the impending revision of the NICE guidelines.[297]
Continued campaigning
Mary Dimmock wrote a comprehensive report Clinical Guidance for ME: “Evidence-Based” Guidance Gone Awry in January 2018 that details how the PACE trial and similar studies based on flawed science led to evidence-based reviews and clinical guidance recommending harmful treatments for ME patients.[298][299]
ME sufferers campaigned against the PACE trial in the Millions Missing protests around the world in 2018, and about the adverse effects of the trial on on biomedical research. As part of this a 'Song for ME: Blowin in the Wind' (available on YouTube) was recorded by ME patients from 7 countries around the world which included the lyrics "How many times must an idea fail...Before it is seen to be flawed?...And how many flaws can a trial embrace...Before it is seen as a fraud?...Yes and how many wounds must its victims expose... Before they’re no longer ignored?".[300]
Dr Racaniello sent another open letter with nearly 100 signatories including scientists, clinicians, academics, lawyers and other experts to the editor Richard Horton at the Lancet due to receiving no response two years later to the original letter from February 2016. The letter again asked for an independent re-analysis from independent reviewers outside UK psychiatry who have no conflicts of interests involving the PACE investigators and the funders of the trial.[301] Another letter of appeal was sent with signatories including 65 ME patient organisation / charities and also this time with 10 Members of Parliament signing the open letter to Richard Horton and the Lancet.[302] The Times (UK) reported on the third letter in its article Call for review of 'flawed' ME research in Lancet letter and stated at the end, "The Lancet declined to comment".[303] The BMJ who were supportive of the PACE trial previously reported it as 'Pressure grows on Lancet to review “flawed” PACE trial' and also approached the Lancet for comment.[304] In a letter to the editor of The Times, the Chief Executive of the MRC, Fiona Watt continued to defend the PACE trial.[303][305]
Additional Scientific Publications[edit | edit source]
BMC Psychology - Re-analysis by Wilshire et al[edit | edit source]
BMC Psychology published on March 22nd, 2018 Rethinking the treatment of chronic fatigue syndrome—A reanalysis and evaluation of findings from a recent major trial of graded exercise and CBT by Carolyn Wilshire, David Tuller, Tom Kindlon, Alem Matthees, Keith Geraghty, Robert Courtney and Bruce Levin.[306] It concluded these findings raise serious concerns about not only the PACE trial but the robustness of the claims made about the efficacy of CBT and GET as a whole. One of the early critics and author, Robert Courtney, died shortly before the paper was published.[306]
Once again, the Science Media Centre on the eve of the publication organised three media briefings for journalists and news organisations, and published a CFS/ME factsheet plus two briefings in what was seen as an attempt to reduce the impact of the publication and spin the story.[307][308] One of the patient authors of the PACE reanalysis asked other patients to contact journalists to ensure they cover the publication and to ensure they have the full facts.[309] Despite the pressure and Science Media Centre briefings, the media did report on PACE trial reanalysis. The Times reported it as Findings of £5m ME chronic fatigue study 'worthless'. Even the publicy-funded news organisation, the BBC, reported on it with Chronic fatigue trial results 'not robust', new study says. The Daily Mail, Huffington Post and other UK national and local media reported on the story.[310][311][312] The news was reported in Europe by Health Europa.[313] The Canary's Steve Topple published in-depth coverage of the reanalysis paper.[314] The NEJM JWatch publicised this to physicians.[315] The European Health Journal also republished the paper.[316]
In their correspondence to BMC Psychology published on March 12, 2019 PACE trial authors Michael Sharpe, Kimberley Goldsmith and Trudie Chalder responded to the re-analysis by Wilshire et al, with The PACE trial of treatments for chronic fatigue syndrome: a response to Wilshire et al. They restated that "both CBT and GET, when given appropriately as supplements to specialist medical care, are more effective at improving both fatigue and physical functioning in people with CFS, than are APT or SMC alone." and that they found "no good reason" to change the conclusions of the PACE trial.[317] Wilshire and Kindlon also responded in BMC Psychology with 'Response: Sharpe, Goldsmith and Chalder fail to restore confidence in the PACE trial findings.[318] In the rejoinder Wilshire and Kindlon clarify the misconception in the PACE trial author’s commentary, and address the seven additional arguments they raise in defence of their conclusion. They concluded "New arguments presented by Sharpe et al. inspire some interesting reflections on the scientific process, but they fail to restore confidence in the PACE trial’s original conclusions." It was also stated that unjustified optimism of CBT/GET fuelled by the PACE trial has caused many consequences including hindering the search for effective treatments and that the debate around the PACE trial will have implications reaching far and beyond.
Other journals[edit | edit source]
Also in March 2018, the British Journal of Healthcare Management published a summary of the controversy over the decades and the scandal over the last two years.[319]
In July 2018, scientists from the Workwell Foundation, University of the Pacific, California, published an editorial in the British Journal of Sports Medicine Checking our blind spots: current status of research evidence summaries in ME/CFS, and concluded "the UK PACE Trial has exposed some of the most important public health-related ethical and legal challenges of our time, which, as yet, remain largely under-reported and unknown to many clinicians." It was supported citizen scientists in its conclusion "We should follow the example of engaged patient advocates and citizen scientists in ME/CFS by involving patients as advisory board members on trials and as reviewers on published evidence summaries....By including evidence from patients in clinical decisions and research, we can begin to address the important blind spots in how we think about and implement EBP."[320]
'PACE-Gate': When clinical trial evidence meets open data access[edit | edit source]
In November 2016, Keith Geraghty published an editorial in the Journal of Health Psychology describing the PACE trial controversy.[321] Geraghty stated:
- "Patients claim the lead authors overstated the effectiveness of cognitive behavioural therapy and graded exercise therapy by lowering the thresholds they used to determine improvement. In this extraordinary case, patients discovered that the treatments tested had much lower efficacy after an information tribunal ordered the release of data from the PACE trial to a patient who had requested access using a freedom of information request".[321]
- Nov 1, 2016, 'PACE-Gate': When clinical trial evidence meets open data access - Keith Geraghty
Textbooks[edit | edit source]
In March 2018, the book 'Health Psychology: Theory, Research and Practice' by Dr David Marks et al. documented the numerous flaws in the PACE trial in a section called The PACE trial: A Catalogue of Errors.[322]
In August 2018 the book Psychology in Crisis by Prof Brian Michael Hughes also documented and summarised the failures of the PACE trial.[323]
Complaint to UK medical regulator (GMC)[edit | edit source]
Dr Sarah Myhill has complained to the UK's medical regulator, the General Medical Council, over the conduct of the PACE trial authors.[324] Over 200 letters of complaints by ME patients were also sent to the General Medical Council. A petition gathered over 8,900 signatories supporting Dr Myhill's complaint to the GMC about the PACE trial authors.[325]
Further FOIA requests[edit | edit source]
Further Freedom of Information Act 2000 requests have been made to the PACE trial team at Queen Mary University of London (QMUL), including a request for a copy of the minutes of the PACE trial management group meetings.[326] The PACE authors continued to refuse to release the PACE trial meeting minutes and other data using various reasons including refusing an internal review and these decisions were appealed again to the Information Commissioner's Office. On February 20, 2018 the Information Commissioner's Office ruled that the the university should release the PACE Trial Steering Committee minutes and the Trial Management Group minutes. The PACE team and the university surprisingly repeated its earlier arguments to the ICO that the criticism is unwarranted and the trial is not controversial among the majority of scientists in the field and that there is a history of the researchers being personal abused, their integrity being questioned and even as far as saying they may risk threats of physical violence. The Information Tribunal in August 2016 in its court decision rejected these serious but unfounded assertions. The complainant, an ME sufferer, argued that this data should be released given the previous submissions by the PACE authors were unreliable and in the intervening period the scientific community in addition to patients had cast further concerns about the trial. The Commissioner found that the public interest favors publishing the anonymized information and ruled that the university should release the minutes of the PACE trial meetings, and had 35 days to do so.[326]
Second Parliamentary debate on PACE Trial[edit | edit source]
It was announced on February 6, 2018 by Carol Monaghan MP that a Westminster Hall debate would be held on the PACE trial called 'PACE trial and its effect on people with ME'.[327] This was exactly five years to the date of the last PACE trial debate in 2013.[328]
The PACE Trial and its effect on people with ME debate took place on February 20, 2018 at Westminster Hall.[329] The debate was concluded by Carol Monaghan MP "I think that when the full details of the trial become known, it will be considered one of the biggest medical scandals of the 21st century."[329]
ME charities Invest in ME Research, ME Association and others commented on the debate.[330][331]
ME advocates have produced an analysis of Caroline Dinenage MP (Department of Health and Social Care) who responded on behalf of the government with mixed and inconsistent responses but continued support for the PACE trial..[332]
An Early Day Motion (EDM) 1247 was launched by Carol Monaghan MP in May for ME Awareness week which stated amongst other points "That this House recognises Myalgic Encephalomyelitis (ME) Awareness Week from 6 to 12 May 2018....acknowledges the detrimental effect of the PACE trial and its results, and the work which is being done to reverse this".[333] It was supported by 136 MPs and was in the top 1% (No 7/1503 as at July 2018) of all EDM's in the parliamentary session.
A further debate on 'M.E. Treatment and Research' was led by Carol Monaghan MP on 21 June 2018 and the PACE trial was again criticised by many MPs.
Quotes from PACE trial critics[edit | edit source]
The PACE trial received extensive criticism from a range of experts. For a selection of quotes from critics about the PACE trial, see the article:
Principal investigators and researchers[edit | edit source]
The principal investigators on the PACE trial are Professors Peter D. White, Trudie Chalder and Michael Sharpe. Additional authors on the 2011 Lancet paper of the PACE Trial Management Group are Kimberley Goldsmith, Anthony L. Johnson, Laura Potts, Rebecca Walwyn, Julia DeCesare, Hannah L. Baber, Mary Burgess, Lucy V. Clark, Diane L. Cox, Jessica Bavinton, Brian Angus, Gabrielle Murphy, Maurice Murphy, Hazel O'Dowd, David Wilks, and Paul McCrone.
The PACE Trial Steering Committee members included Mansel Aylward.
List of PACE trial publications[edit | edit source]
2003: Trial Registry. BioMed Central. ISRCTN54285094
There is a currently published trial register and there is a version of the register that is no longer available on the web but has been archived.[334][335] Both versions contain slightly different details. The archived version contains the details of the trial's pre-specified endpoint analyses.
2006: Final protocol version 5.0
This full version of the study protocol, which includes trial materials such as questionnaires and consent forms, was never officially published.[336]
2007: PACE trial protocol
BMC Neurology published the trial's planned methodology, including its planned analyses.[13]
2011: Main trial outcomes
The trial's main outcomes, and some selected secondary outcomes, were reported in The Lancet.[2] Forty-four letters were submitted in a response that the journal's editor described as "swift and damning".[178]
The Science Media Centre organised a press briefing and Expert Opinion on ME/CFS Study, on Feb 17, 2011.[337] The UK and international media heavily publicised this paper after the PACE authors' press conference and in conjunction with the Science Media Centre in February 2011.[25]
2012: Cost-effectiveness of CBT and GET
This paper was published in the PLoS One journal and concluded that cognitive behavioral therapy and graded exercise therapy had the best probability of being the most cost-effective treatments.[20] The UK media publicised the results from this paper[352][353][354][355][356] Controversy has broken out over the failure of the study authors to provide the underlying data to Professor James Coyne under PLoS One's data-sharing policy.[196] Journalist David Tuller has criticized the study's assumptions and conclusions.[357]
2013: "Recovery" rates
This paper appeared in Psychological Medicine.[3] The Science Media Centre issued an "expert opinion" about the study.[271]
ME/CFS patient Graham McPhee and others created a video animation - How's that recovery? - explaining problems with the new analyses that had replaced those specified in the study protocol. Sam Carter applied the new fatigue and physical function recovery criteria to the data from the FINE trial and found that doing so increased the number of "recovered" patients six-fold, compared to the original criteria.[358]
Patient-advocate Peter Kemp also criticized the study, stating that the authors had "twisted the SF-36 Physical Function subscale to suit their needs."[359]
Patients created a tongue-in-cheek song video to satirically criticize the recovery paper results.[360]
2013: Statistical analysis plan
Trials Journal published the detailed plan for PACE's data analysis.[361]
2014: Adverse effects
The Journal of Psychosomatic Research published the results on safety and adverse effects.[72]
2015: Secondary mediation analysis
The Lancet Psychiatry published this paper. [21] The UK and international media publicised the results from this paper.[40][41][362][363][364][365][42] [366][367][368][369]
The paper has been criticised by Byron Hyde.[370]
2015: Long-term follow-up
Long-term follow-up results were published in The Lancet Psychiatry in October 2015.[4]
The paper states that at least two years after patients were randomised, "there were no significant differences" in outcomes between the treatment groups. This indicated that APT, CBT and GET added nothing to specialist medical care (SMC), which all groups received. However, the study authors interpreted the results as favouring CBT and GET.
The Science Media Centre published "expert reaction" to the paper.[371] The UK media then publicised the results. [36][37][38][39][42][44] [372][373]
Professors James Coyne and Keith Laws criticised the results as "uninterpretable" and consisting of "null findings."[71] The ME Association also criticized the follow-up study.[374]
2015: Longitudinal mediation analysis
This short paper published in Trials Journal concluded: "Approximately half of the effect of each of CBT and GET [...] on physical function was mediated through reducing avoidance of fearful situations".[375]
Other publications[edit | edit source]
- 2014: Pain in chronic fatigue syndrome: response to rehabilitative treatments in the PACE trial
- 2013: The planning, implementation and publication of a complex intervention trial for chronic fatigue syndrome: the PACE trial[376]
- 2013: Cognitions, behaviours and co-morbid psychiatric diagnoses in patients with chronic fatigue syndrome
- 2013: Training, Supervision & Therapists' Adherence to Manual Based Therapies
- 2011: = 21843745 Measuring disability in patients with chronic fatigue syndrome: reliability and validity of the Work and Social Adjustment Scale
- 2010: Psychiatric misdiagnoses in patients with chronic fatigue syndrome
Talks and interviews[edit | edit source]
- 2011, Michael Sharpe interview
- 2011: Professors Michael Sharpe and Trudie Chalder were videoed[22][23][24] at The Lancet's press conference for the publication of the trial's main results in 2011.
- 2011: "Health Report - Comparison of treatments for chronic fatigue syndrome - the PACE trial", ABC Radio National (Australia), 18 Apr 2011[179]
- 2015: Professor James Coyne gave a talk on "A skeptical look at the PACE chronic fatigue trial" in Edinburgh,[103][104][105] transcript,[377] and slides[106] available online) and, more recently, Belfast[107] and slides [108]
- 2016: While in Amsterdam at The Forgotten Plague Conference, David Tuller gave an = youtu.be interview with Frank Twisk on 27 Feb 2016.[378] The following day, Feb 28, 2016, David Tuller gave a speech with Q&As.[379]
- June 2017: = pl%2Cwn #TearItUp PACE trial Invest in ME Pre-Conference dinner talk by Dr David Tuller
- September 2018: How Not To Conduct A Randomized Clinical Trial by Dr Bruce Levin, Ph.D., Department of Biostatistics, Mailman School of Public Health, Columbia University Irving Medical Center
- October 2018: 'The PACE Trial: ‘One of Greatest Medical Scandals of the 21st Century' talk by Dr David Tuller and Professor Brian Hughes, Newry, Northern Ireland
See also[edit | edit source]
- PACE trial documents
- Intimidation and bullying of PACE trial critics
- Open letter to the Lancet
- An open letter to Psychological Medicine about “recovery” and the PACE trial
- List of scientific and media response to release of PACE trial data
- Action for ME
- Cochrane
- Cognitive behavioral therapy
- Graded exercise therapy
- FINE trial - PACE's "sister trial"
- Journal of Health Psychology, Special Issue: The PACE Trial
- Quotes from critics of the PACE trial
- NICE guidelines
- PACE Trial Management Group
- PACE Trial Steering Committee
- Research bias in ME/CFS
- PACE trial critics
- Proponents of the psychological theory of ME/CFS
- Psychological paradigm critics
- The Lancet
- The Lancet Psychiatry
Learn more[edit | edit source]
- Twitter # : #PACEtrial #PACEgate
- Co-Cure Listserve (from 2003): PACE trial
- Phoenix Rising Forum: PACE trial
- Science for ME Forum: PACE trial
- Pace Trial Website
References[edit | edit source]
- ↑ 1.0 1.1 White, Peter D.; Sharpe, Michael C.; Chalder, Trudie; DeCesare, Julia C.; Walwyn, Rebecca; PACE trial group (March 8, 2007). "Protocol for the PACE trial: a randomised controlled trial of adaptive pacing, cognitive behaviour therapy, and graded exercise, as supplements to standardised specialist medical care versus standardised specialist medical care alone for patients with the chronic fatigue syndrome/myalgic encephalomyelitis or encephalopathy". BMC neurology. 7: 6. doi:10.1186/1471-2377-7-6. ISSN 1471-2377. PMC 2147058. PMID 17397525.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 White, PD; Goldsmith, KA; Johnson, AL; Potts, L; Walwyn, R; DeCesare, JC; Baber, HL; Burgess, M; Clark, LV; Cox, DL; Bavinton, J; Angus, BJ; Murphy, G; Murphy, M; O'Dowd, H; Wilks, D; McCrone, P; Chalder, T; Sharpe, M; The PACE Trial Management Group (March 5, 2011). "Comparison of adaptive pacing therapy, cognitive behaviour therapy, graded exercise therapy, and specialist medical care for chronic fatigue syndrome (PACE): a randomised trial". The Lancet. 377 (9768): 823–836. doi:10.1016/S0140-6736(11)60096-2. PMID 21334061.
- ↑ 3.0 3.1 3.2 White, PD; Goldsmith, K; Johnson, AL; Chalder, T; Sharpe, M; PACE Trial Management Group (October 2013). "Recovery from chronic fatigue syndrome after treatments given in the PACE trial". Psychol Med. 43 (10): 2227–2235. doi:10.1017/S0033291713000020. PMID 3776285.
- ↑ 4.0 4.1 4.2 4.3 4.4 Sharpe, M; Goldsmith, KA; Johnson, AL; Chalder, T; Walker, J; White, PD (October 27, 2015), "Rehabilitative treatments for chronic fatigue syndrome: long-term follow-up from the PACE trial", The Lancet Psychiatry, 2: 1067-74, doi:10.1016/S2215-0366(15)00317-X, PMID 26521770,
There was little evidence of differences in outcomes between the randomised treatment groups at long-term follow-up.
- ↑ 5.0 5.1 #MEAction (October 2015), "Petition: Misleading Claims Should Be Retracted", The MEAction Network, archived from the original on March 19, 2019
- ↑ 6.0 6.1 ME Association (May 29, 2015), Our CBT, GET and Pacing Report calls for major changes to therapies offered for ME/CFS
- ↑ 7.0 7.1 7.2 7.3 Davis, Ronald W; Edwards, Jonathan C W; Jason, Leonard A; Levin, Bruce; Racaniello, Vincent R; Reingold, Arthur L (November 13, 2015). "An open letter to Dr Richard Horton and The Lancet". Virology blog.
- ↑ Gallagher, Paul (August 19, 2016). "Chronic Fatigue Syndrome: tribunal orders data from controversial trial to be released". iNews UK.
- ↑ 9.0 9.1 9.2 9.3 NICE guidelines committee (August 2007), NICE guidelines (CG53) - Chronic fatigue syndrome / myalgic encephalomyelitis (or encephalopathy): diagnosis and management
- ↑ 10.0 10.1 White, Peter D (2011), "Managing claims for chronic fatigue the active way", Swiss Re (insurance), archived from the original on July 25, 2013
- ↑ 11.0 11.1 White, Peter D (2011). "Managing claims for chronic fatigue the active way". Swiss Re (insurance). Archived from the original on August 24, 2013.
- ↑ University of Edinburgh (2008), RA5a: Research environment and esteem
- ↑ 13.0 13.1 13.2 White, PD; Sharpe, MC; Chalder, T; DeCesare, JC; Walwyn, R; The PACE trial group (March 8, 2007), "Protocol for the PACE Trial: A randomised controlled trial of adaptive pacing, cognitive behaviour therapy, and graded exercise as supplements to standardised specialist medical care versus standardised specialist medical care alone for patients with the chronic fatigue syndrome/myalgic encephalomyelitis or encephalopathy", BMC Neurology, doi:10.1186/1471-2377-7-6, PMID 17397525
- ↑ 14.0 14.1 14.2 Wessely, Simon (November 4, 2015). "The PACE Trial for chronic fatigue syndrome: choppy seas but a prosperous voyage". National Elf Service.
- ↑ PACE Trial Management Group (December 2, 2004), PACE - Manual for Doctors - Standardised Specialist Medical Care (SSMC) (PDF)
- ↑ 16.0 16.1 PACE Trial Management Group (November 2004), PACE - Manual for Participants - Adaptive Pacing Therapy (APT) for CFS/ME (PDF)
- ↑ 17.0 17.1 PACE Trial Management Group (November 2004), PACE - Manual for Participants - Cognitive Behaviour Therapy for CFS/ME (PDF)
- ↑ 18.0 18.1 PACE Trial Management Group (November 2004), PACE - Manual for Participants - Graded Exercise Therapy for CFS/ME (PDF)
- ↑ "PACE Trial". Queen Mary University Of London. Retrieved July 16, 2022.
- ↑ 20.0 20.1 20.2 McCrone, P; Sharpe, M; Chalder, T; Knapp, M; Johnson, AL; Goldsmith, K (August 1, 2012). "Adaptive Pacing, Cognitive Behaviour Therapy, Graded Exercise, and Specialist Medical Care for Chronic Fatigue Syndrome: A Cost-Effectiveness Analysis". PLoS One. doi:10.1371/journal.pone.0040808. PMID 22870204.
- ↑ 21.0 21.1 21.2 Chalder, T; Goldsmith, KA; White, PD; Sharpe, M; Pickles, AR (January 28, 2015), "Rehabilitative therapies for chronic fatigue syndrome: a secondary mediation analysis of the PACE trial", The Lancet Psychiatry, 2 (2): 141–52, doi:10.1016/S2215-0366(14)00069-8, PMID 26359750
- ↑ 22.0 22.1 Sharpe, M; Chalder, T (February 17, 2011), "London Press Conference: Chronic Fatigue Syndrome - part 1 (video)", The Lancet TV
- ↑ 23.0 23.1 Sharpe, M (February 17, 2011), "London Press Conference: Chronic Fatigue Syndrome - part 2 (video)", The Lancet TV
- ↑ 24.0 24.1 Chalder, T (February 17, 2011), "London Press Conference: Chronic Fatigue Syndrome - part 3 (video)", The Lancet TV
- ↑ 25.0 25.1 Boseley, Sarah (February 18, 2011). "Study finds therapy and exercise best for ME". The Guardian.
- ↑ 26.0 26.1 26.2 26.3 Tuller, David (October 21, 2015). "Trial by Error: The Troubling Case of the PACE Chronic Fatigue Syndrome Study - Part 1". Virology blog.
- ↑ 27.0 27.1 Tuller, David (October 22, 2015). "Trial by Error: The Troubling Case of the PACE Chronic Fatigue Syndrome Study - Part 2". Virology blog.
- ↑ 28.0 28.1 Tuller, David (October 23, 2015). "Trial by Error: The Troubling Case of the PACE Chronic Fatigue Syndrome Study - Part 3". Virology blog.
- ↑ 29.0 29.1 Reuters (February 17, 2011), Pushing limits can help chronic fatigue patients
- ↑ 30.0 30.1 WebMD (US) (February 17, 2011). "Study Shows Cognitive Behavioral Therapy, Exercise Are Safe Ways to Treat CFS Symptoms".
- ↑ 31.0 31.1 31.2 "Brain and body training treats ME, UK study says". BBC News. February 18, 2011.
- ↑ 32.0 32.1 CNN News (US) (February 18, 2011). "Study supports use of 2 controversial treatments for chronic fatigue".
- ↑ Medical News Today (February 1, 2013), Chronic Fatigue Treatments Lead To Recovery In Trial
- ↑ "Got ME? Fatigued patients who go out and exercise have best hope of recovery, finds study". Daily Mail. February 18, 2011.
- ↑ 35.0 35.1 New York Times (February 17, 2011). "Psychotherapy Eases Chronic Fatigue Syndrome, Study Finds".
- ↑ 36.0 36.1 "Exercise and positive thinking 'can help overcome' Chronic Fatigue Syndrome". Evening Standard. October 28, 2015.
- ↑ 37.0 37.1 "Chronic Fatigue Syndrome sufferers 'can overcome symptoms of ME with positive thinking and exercise' / Oxford University has found ME is not actually a chronic illness". Independent (IE). October 28, 2015.
- ↑ 38.0 38.1 Torjesen, Ingrid (October 28, 2015), "Tackling fear about exercise produces long term benefit in chronic fatigue syndrome", The BMJ
- ↑ 39.0 39.1 "ME can be beaten by taking more exercise and positive thinking, landmark study claims". Daily Mail. October 28, 2015.
- ↑ 40.0 40.1 FoxNews (January 14, 2015). "Helping chronic fatigue patients over fears eases symptoms".
- ↑ 41.0 41.1 "Chronic fatigue syndrome patients' fear of exercise can hinder treatment - study". The Guardian. January 14, 2015.
- ↑ 42.0 42.1 42.2 Knapton, Sarah (October 28, 2015). "Chronic Fatigue Syndrome sufferers 'can overcome symptoms of ME with positive thinking and exercise". The Telegraph.
- ↑ #MEAction (October 28, 2015), "PACE trial controversy grows", The MEAction Network
- ↑ 44.0 44.1 Cohen, Jon (October 27, 2015). "Criticism mounts of a long-controversial chronic fatigue study". Science Magazine.
- ↑ MEActionUK (April 16, 2011), The Media and ME
- ↑ Larun, Lillebeth; Brurberg, Kjetil G; Odgaard-Jensen, Jan; Price, Jonathan R (October 2, 2019). Cochrane Common Mental Disorders Group (ed.). "Exercise therapy for chronic fatigue syndrome". Cochrane Database of Systematic Reviews. 2021 (3). doi:10.1002/14651858.CD003200.pub8. PMC 6953363. PMID 31577366.
- ↑ British Association for CFS/ME (BACME) (March 2011), Statement on the PACE Trial results
- ↑ ME Association (October 23, 2013). "MEA opposes plan to put review of NICE ME/CFS Guideline on hold".
- ↑ NICE guideline committee (February 2014), NICE guidelines (CG53) - Chronic fatigue syndrome / myalgic encephalomyelitis (or encephalopathy): review decision
- ↑ National Institute for Clinical Excellence. "Surveillance decision | Chronic fatigue syndrome/myalgic encephalomyelitis (or encephalopathy): diagnosis and management | Guidance and guidelines". nice.org.uk. Retrieved October 19, 2018.
- ↑ US Centers for Disease Control and Prevention (CDC) (June 27, 2012), Diagnosis and Management of Chronic Fatigue Syndrome, retrieved March 10, 2016
- ↑ Egeland, T; Angelsen, A; Haug, R; Henriksen, JO; Lea, TE; Saugstad, OD (October 2015). "What exactly is myalgic encephalomyelitis?". Tidsskr Nor Legeforen. 135 (19): 1756–9. doi:10.4045/tidsskr.15.0089.
- ↑ Jason, Leonard A; McManimen, Stephanie; Sunnquist, Madison; Brown, Abigail; Furst, Jacob; Newton, Julia L; Strand, Elin Bolle (January 2016), "Case definitions integrating empiric and consensus perspectives", Fatigue: Biomedicine, Health & Behavior, 4 (1): 1–23, doi:10.1080/21641846.2015.1124520
- ↑ National Institutes of Health (US) (December 9, 2014), NIH Pathways to Prevention Workshop: Advancing the Research on Myalgic Encephalomyelitis/ Chronic Fatigue Syndrome (PDF)
- ↑ 55.0 55.1 55.2 55.3 Tuller, David (October 30, 2015). "PACE trial investigators respond to David Tuller". Virology blog.
- ↑ 56.0 56.1 56.2 Davis, Ronald W; Edwards, Jonathan C W; Jason, Leonard A; Levin, Bruce; Racaniello, Vincent R; Reingold, Arthur L; Ablashi, Dharam V; Baraniuk, James N; Barcellos, Lisa F; Bateman, Lucinda; Bell, David S; Bested, Alison C; Broderick, Gordon; Chia, John; Chu, Lily; Enlander, Derek; Fletcher, Mary Ann; Friedman, Kenneth; Kaufman, David L; Klimas, Nancy; Lapp, Charles W; Levine, Susan; Light, Alan R; Marshall-Gradisnik, Sonya; Medveczky, Peter G; Nahle, Zaher; Oleske, James M; Podell, Richard N; Shepherd, Charles; Snell, Christopher R; Speight, Nigel; Staines, Donald; Stark, Philip B; Stein, Eleanor; Swartzberg, John; Tompkins, Ronald G; Underhill, Rosemary; Vallings, Rosamund; VanElZakker, Michael; Weir, William; Zinn, Marcie L; Zinn, Mark A (February 10, 2016), "An open letter to the Lancet - again", Virology blog
- ↑ Juenger, J; Schellberg, D; Kraemer, S; Haunstetter, A; Zugck, C; Herzog, W; Haass, M (March 2002), "Health related quality of life in patients with congestive heart failure: comparison with other chronic diseases and relation to functional variables", Heart, 87 (3): 235–241, PMID 1767036
- ↑ 58.0 58.1 Goldin, Rebecca (March 21, 2016), "PACE: The research that sparked a patient rebellion and challenged medicine", Sense About Statistics (American Statistical Association)
- ↑ #MEAction (October 28, 2015), "What's Wrong in the Lancet", The MEAction Network
- ↑ #MEAction (October 28, 2015), "What's Wrong in Psychological Medicine", The MEAction Network
- ↑ 61.0 61.1 Courtney, Robert (October 29, 2013), FOI Request: PACE Recovery Rates and Positive Outcome Rates (repeat request)
- ↑ Matthees, Alem (March 24, 2014), FOI Request: Selected data on PACE Trial participants
- ↑ McPhee, Graham (July 28, 2015), FOI Request: Fitness data for PACE trial
- ↑ 64.0 64.1 Information Commissioner's Office, UK (March 9, 2016), FOI Decision Notice: to QMUL (PDF)
- ↑ Edwards, Jonathan CW (January 18, 2015), "(response) Re: Tackling fears about exercise is important for ME treatment", BMJ, 350: h227
- ↑ 66.0 66.1 Edwards, Jonathan CW (November 1, 2015), "Prof. Jonathan Edwards: PACE trial is 'valueless'", The MEAction Network
- ↑ PACE Trial Management Group (June 2006), PACE trial participants' newsletter #1 (PDF)
- ↑ PACE Trial Management Group (March 2007), PACE trial participants' newsletter #2 (PDF)
- ↑ 69.0 69.1 PACE Trial Management Group (December 2008), PACE trial participants' newsletter #3 (PDF)
- ↑ PACE Trial Management Group (February 2011), PACE trial participants' newsletter #4 (PDF)
- ↑ 71.0 71.1 71.2 Coyne, James (October 29, 2015), Uninterpretable: Fatal flaws in PACE Chronic Fatigue Syndrome follow-up study, PLoS One Blog, archived from the original on May 6, 2016
- ↑ 72.0 72.1 Dougall, D; Johnson, A; Goldsmith, K; Sharpe, M; Angus, B; Chalder, T; White, P (July 2014). "Adverse events and deterioration reported by participants in the PACE trial of therapies for chronic fatigue syndrome". Journal of Psychosomatic Research. 77 (1): 20–26. doi:10.1016/j.jpsychores.2014.04.002.
- ↑ 73.0 73.1 Kindlon, Tom (2011), "Reporting of Harms Associated with Graded Exercise Therapy and Cognitive Behavioural Therapy in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome", Bulletin of the IACFS/ME, 19 (2): 59–111
- ↑ Tuller, David (January 7, 2016). "Trial By Error, Continued: Did the PACE Trial Really Prove that Graded Exercise Is Safe?". Virology blog.
- ↑ Courtney, Robert (April 2015), "(correspondence) Doubts over the validity of the PACE hypothesis", The Lancet Psychiatry, doi:10.1016/S2215-0366(15)00054-1
- ↑ Kemp, Peter (February 2016), PACE Trial Analysis
- ↑ Kennedy, Angela (February 10, 2016). "Summary of my specific concerns about PACE with annotated bibliography". PACE Documents blogspot.
- ↑ Twisk, Frank (January 18, 2016), "(correspondence) PACE: CBT and GET are not rehabilitative therapies", The Lancet Psychiatry, doi:10.1016/S2215-0366(15)00554-4
- ↑ Mitchell, John T, Jnr (May 17, 2011), "(correspondence) The PACE trial in chronic fatigue syndrome", The Lancet
- ↑ Feehan, Sarah M; on behalf of the Liverpool ME Support Group (May 17, 2011), "(correspondence) The PACE trial in chronic fatigue syndrome", The Lancet, doi:10.1016/S0140-6736(11)60688-0
- ↑ Giakoumakis, Jane (May 17, 2011), "(correspondence) The PACE trial in chronic fatigue syndrome", The Lancet, 377 (9780): 1831, doi:10.1016/S0140-6736(11)60689-2
- ↑ Invest in ME (IIME) (November 14, 2015), Letter to the Editor of the Lancet – The PACE Trial (PDF)
- ↑ Colby, Jane (February 24, 2011). "ME – the truth about exercise and therapy". The Guardian.
- ↑ #MEAction (2015). "PRESS RELEASE: 12,000 SIGNATURE PACE PETITION DELIVERED TO THE LANCET". MEAction. Retrieved June 10, 2020.
- ↑ #MEAction (October 28, 2015), "PACE Trial Overview", The MEAction Network
- ↑ #MEAction (May 2015), "Patient view of the PACE trial controversy" (PDF), The MEAction Network
- ↑ Kellgren-Fozard, Jessica (May 7, 2018). "Have you been misled...? // What is PACE? // Medical Scandal". YouTube. Retrieved October 6, 2010.
- ↑ 88.0 88.1 Tuller, David (October 30, 2015). "David Tuller responds to PACE trial investigators". Virology blog.
- ↑ Tuller, David (November 4, 2015). "Trial By Error, Continued: Did the PACE Study Really Adopt a 'Strict Criterion' for Recovery?". Virology blog.
- ↑ Tuller, David (November 9, 2015). "Trial By Error, Continued: Why has the PACE Study's Sister Trial been Disappeared and Forgotten?". Virology blog.
- ↑ Tuller, David (November 17, 2015). "Trial By Error, Continued: PACE Team's Work for Insurance Companies Is "Not Related" to PACE. Really?". Virology blog.
- ↑ Tuller, David (January 4, 2016). "Trial By Error, Continued: Questions for Dr. White and his PACE Colleagues". Virology blog.
- ↑ Hooper, Malcolm; Williams, Margaret (June 20, 2004), "Issues re the use of the Oxford criteria for the MRC "CFS" Trials" (PDF), MargaretWilliams
- ↑ Hooper, Malcolm; Williams, Margaret (December 17, 2009), "Can the MRC PACE Trial be justified" (PDF), MargaretWilliams
- ↑ 95.0 95.1 Hooper, Malcolm (February 2010). "Magical Medicine: How to Make a Disease Disappear" (PDF).
- ↑ Hooper, Malcolm; Williams, Margaret (March 2011), "Complaint to the Editor of the Lancet about the PACE Trial Articles" (PDF), MargaretWilliams
- ↑ 97.0 97.1 Hooper, Malcolm; Williams, Margaret (May 28, 2011), "Professor Malcolm Hooper's Detailed Response to Professor Peter White's letter to Dr Richard Horton about his complaint re: the PACE Trial articles published in The Lancet" (PDF), MargaretWilliams
- ↑ Hooper, Malcolm; Williams, Margaret (June 4, 2011), "Professor Malcolm Hooper's further concerns about the PACE Trial article published in The Lancet" (PDF), MargaretWilliams
- ↑ Hooper, Malcolm; Williams, Margaret (September 2013), "The Role of the Science Media Centre and the Insurance Industry in ME/CFS: the facts behind the fiction" (PDF), MargaretWilliams
- ↑ Hooper, Malcolm; Williams, Margaret (September 2013), "Key Concerns about the PACE trial" (PDF), MargaretWilliams
- ↑ Hooper, Malcolm; Williams, Margaret (November 14, 2015), "PACE Trial Key Dates and Chronology of Complaint" (PDF), MargaretWilliams
- ↑ List of ME/CFS articles published at Virology Blog
- ↑ 103.0 103.1 Coyne, James (November 16, 2015), "A skeptical look at the PACE chronic fatigue trial - video part 1", YouTube
- ↑ 104.0 104.1 Coyne, James (November 16, 2015). "A skeptical look at the PACE chronic fatigue trial - video part 2". YouTube.
- ↑ 105.0 105.1 Coyne, James (November 16, 2015), "A skeptical look at the PACE chronic fatigue trial - video part 3", YouTube
- ↑ 106.0 106.1 Coyne, James (November 16, 2015), "A skeptical look at the PACE chronic fatigue trial - slide show", Slideshare
- ↑ 107.0 107.1 Coyne, James (February 8, 2016). "The scandal of the £5m PACE trial for ME - video".
- ↑ 108.0 108.1 Coyne, James (February 8, 2016), The scandal of the £5m PACE trial for ME - slide show
- ↑ Coyne, James (November 25, 2015). "Was independent peer review of the PACE trial articles possible?". MindTheBrain Blog.
- ↑ Coyne, James. "ME/CFS". Quick Thoughts blog.
- ↑ Laws, Keith (November 1, 2015). "PACE - Thoughts about Holes". LawsDystopia Blog.
- ↑ Laws, Keith (November 6, 2015). "PACE - Song for the Siren". LawsDystopia Blog.
- ↑ Coyne, James; Laws, Keith (February 18, 2016), "(comment) Results of the PACE follow-up study are uninterpretable", The Lancet Psychiatry, 3 (2): e6–e7
- ↑ Rehmeyer, Julie (November 13, 2015). "Hope for Chronic Fatigue Syndrome". Slate.
- ↑ 115.0 115.1 Rehmeyer, Julie; Tuller, David (March 18, 2017). "Getting It Wrong on Chronic Fatigue Syndrome". The New York Times, Sunday Review.
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs named<ref name= - ↑ Bailey, Lucy (January 27, 2016), "My thoughts about the PACE trial", Lucibee's Blog
- ↑ Bailey, Lucy (October 2017), "A Case for Retraction ?", The Lancet Infectious Diseases, doi:10.1016/S1473-3099(17)30527-3
- ↑ Bailey, Lucy (May 9, 2018). "PACE trial: Whatever happened to actigraphy?". Lucibee's Blog.
- ↑ Vink, Mark (March 30, 2016). "The PACE Trial Invalidates the Use of Cognitive Behavioral and Graded Exercise Therapy in Myalgic Encephalomyelitis/ Chronic Fatigue Syndrome: A Review". J Neurol Neurobiol. 2 (3). doi:10.16966/2379-7150.124.
- ↑ Schneider, Leonid (April 5, 2016). "Does The Lancet care about patients?". ForBetterScience Blog.
- ↑ 122.0 122.1 Schneider, Leonid (February 8, 2016). "PACE trial and other clinical data sharing: patient privacy concerns and parasite paranoia". ForBetterScience Blog.
- ↑ Schneider, Leonid. "Index of PACE Trial articles". ForBetterScience Blog.
- ↑ 124.0 124.1 Gelman, Andrew (December 23, 2015), "To keep science honest, study data must be shared", StatNews
- ↑ Gelman, Andrew (December 25, 2015), "More on the PACE (chronic fatigue syndrome study) scandal", Statistical Modeling, Causal Inference, and Social Science (blog)
- ↑ Gelman, Andrew (January 5, 2016), "PACE study and the Lancet: Journal reputation is a two-way street", Statistical Modeling, Causal Inference, and Social Science (blog)
- ↑ Gelman, Andrew (July 9, 2017). "A Memoir of Chronic Fatigue Illustrates the Failures of Medical Research". New Yorker.
- ↑ Helmfrid, Sten (September 2016), "Studies on Cognitive Behavioral Therapy and Graded Exercise Therapy for ME/CFS are misleading", Socialmedicinsk tidskrift
- ↑ Lee, Sonia (February 23, 2017), "PACE Trial: Critical Appraisal (slides)", Figshare, doi:10.6084/m9.figshare.4685074.v1
- ↑ Lee, Sonia (February 23, 2017). "PACE Trial: Critical Appraisal (video)".
- ↑ Lee, Sonia (May 7, 2017). "Research Waste in ME/CFS". biorxiv. doi:10.1101/133926.
- ↑ Jason, Leonard A (February 2017), "The PACE trial missteps on pacing and patient selection", Journal of Health Psychology, 22 (9): 1141–1145, doi:10.1177/1359105317695801
- ↑ Myhill, Sarah (August 22, 2017), "MAIMES (Medical Abuse in ME Sufferers) - The Time Has Come To End The Abuse", YouTube
- ↑ "Prominent Australian ME/CFS researcher, Neil McGregor, has weighed in on the GET debate". Facebook. ME Advocacy Network Australia. June 6, 2017.
- ↑ McGregor, Neil (2017). "Harm and the PACE trial" (PDF). Dropbox.
- ↑ Anderssen, Alex (September 5, 2016). "Lancet Rejects Scientists' PACE Letter". The MEAction Network.
- ↑ Racaniello, Vincent (August 21, 2016), "TWiV 403: It's not easy being vaccine", This Week In Virology (podcast)
- ↑ 138.0 138.1 Murray, Robin; Agardy, Susanna; Carter, Samuel; Courtney, Robert; Cox, Duncan; Maryhew, Carly; Shepherd, Charles; White, Peter (July 22, 2013). "Letter to the Editor: Comments on 'Recovery from chronic fatigue syndrome after treatments given in the PACE trial'". Journal of Psychological Medicine. 43 (8): 1787–1787. doi:10.1017/S003329171300113X.
Editorial note
Unusually for Psychological Medicine, we publish below six letters concerning the paper by White et al (2013) on the PACE Trial. The UK Office of the Journal received 15 letters criticising aspects of this paper, but it seemed unlikely that all of these letters originated entirely independently since a number arrived on consecutive days and reiterated the same points. Nevertheless, in the spirit of scientific openness, we have published six of the letters which cover the main criticisms, and invited Professor White to reply to them. - ↑ "PACE trial: letters and reply | Journal of Psychological Medicine | August 2013". ME Association. August 23, 2013.
- ↑ 140.0 140.1 European ME Alliance (EMEA); ME-Vereniging (Belgium); Foreningen for Myalgisk Encefalomyelitis (Denmark); ME Foreningen (Denmark); Suomen CFS-Yhdistys (Finland); Fatigatio e.V. (Germany); Het Alternatief (Netherlands); ME félag Íslands (Iceland); Irish ME Trust (Ireland); Associazione Malati di CFS (Itali); Norges ME-forening (Norway); Liga SFC (Spain); Riksföreningen för ME-patienter (RME) (Sweden); Verein ME/CFS Schweiz (Switzerland); Invest in ME Research (UK) (March 12, 2016). "Open Letter to QMUL - Request for the release of PACE trial data" (PDF).
- ↑ #MEAction (March 2016), "#MEAction delivers Lancet petition, makes Wall Street Journal", The MEAction Network
- ↑ #MEAction (November 16, 2015), "Call for HHS to Investigate Pace", The MEAction Network
- ↑ Hale, Catherine (May 12, 2017). "#MillionsMissing protest London 12.05.17". Retrieved October 6, 2022.
- ↑ White, PD (March 31, 2006), Letter from Professor Peter White re Theft of patient data (PDF)
- ↑ "Centre for Welfare Reform criticises PACE trial". The MEAction Network. April 26, 2016. Retrieved October 6, 2022.
- ↑ Duffy, Simon; Centre for Welfare Reform (UK) (April 20, 2016). "The Misleading Research at the Heart of Disability Cuts". Huffington Post (UK).
- ↑ Butterworth, Trevor (March 21, 2016), "Editorial: On PACE", Sense About Statistics(American Statistical Association)
- ↑ #MEAction (August 8, 2016). "Rehmeyer makes statisticians' jaws drop over PACE". The MEAction Network.
- ↑ UK Parliament (February 6, 2013), UK House of Lords, Debates the PACE Trial - 6 February 2013 (video)
- ↑ UK Parliament (February 6, 2013), UK House of Lords, Debates the PACE Trial - 6 February 2013 (transcript)
- ↑ Hooper, Malcolm; Williams, Margaret (February 6, 2013), "Comments on the PACE debate held in House of Lords (Grand Committee) on 6th February 2013" (PDF), MargaretWilliams
- ↑ Hopkins, Kelvin (2016). "Find written questions and answers". UK Parliament - Written questions, answers and statements. Retrieved October 6, 2010.
- ↑ Hooper, Malcolm; Williams, Margaret (February 1, 2017), "Concerns about Public Funding for the PACE Trial - Committee of Public Accounts" (PDF), MargaretWilliams
- ↑ White, PD; Goldsmith, KA; Johnson, AL; Walwyn, R; Baber, HL; Chalder, T; Sharpe, M (May 17, 2011). "(correspondence) The PACE trial in chronic fatigue syndrome – Authors' reply". The Lancet. 377 (9780): 1834–1835. doi:10.1016/S0140-6736(11)60651-X.
- ↑ Chalder, T; Goldsmith, KA; White, PD; Sharpe, M; Pickles, AR (April 2015), "(response) Author's reply - Methods and outcome reporting in the PACE trial", The Lancet Psychiatry, 2 (4): e10–e11, doi:10.1016/S2215-0366(15)00114-5
- ↑ Sharpe, M; Goldsmith, KA; Johnson, AL; Chalder, T; Walker, J; White, PD (February 2016). "(correspondence) Authors' reply - Patient reaction to the PACE trial". The Lancet Psychiatry. 3 (2): e8–e9. doi:10.1016/S2215-0366(16)00018-3.
- ↑ Kindlon, Tom (August 30, 2013), "(correspondence) Re: College was right not to disclose deliberations about chronic fatigue treatment trial, tribunal rules", BMJ, doi:10.1136/bmj.f5355
- ↑ White, PD (September 25, 2013). "(response) Re: People want to learn as much as possible from the PACE trial for chronic fatigue syndrome". BMJ. pp. f5731. doi:10.1136/bmj.f5731.
- ↑ (various authors) (October 15, 2013), "Re: PACE trial authors' reply to letter by Kindlon", The BMJ (347): f5963, doi:10.1136/bmj.f5963
- ↑ James Coyne [@coyneoftherealm] (November 4, 2015). "@Mental_Elf asked me to join PACE investigators in live debate. I agreed, they did not" (Tweet) – via Twitter.
- ↑ McKie, Robin (August 21, 2011). "Chronic fatigue syndrome researchers face death threats from militants". The Guardian.
- ↑ Wessely, Simon (August 27, 2011). "Mind the gap - It's time to stop separating psychiatry and neurology". Spectator (UK).
- ↑ Wessely, Simon (July 29, 2011). "Malicious' harassment of ME researchers". BBC - Today.
- ↑ Zimmer, Carl (August 21, 2011). "Chronic Fatigue Syndrome: Death threats for scientists?". Discover Magazine.
- ↑ Lowe, Derek (September 6, 2011). "Chronic Fatigue: Enough Energy Left for Death Threats, Anyway". Science Translational Medicine Blog.
- ↑ "ME researchers 'receive death threats from sufferers'". The Telegraph. July 29, 2011.
- ↑ "ME/CFS: Harassment of Researchers", Stuff and Nonsense Blog, November 17, 2012
- ↑ Fielden, Tom (July 21, 2011). "'Torrent of abuse' hindering ME research". BBC News.
- ↑ Hawkes, Nigel (July 22, 2011). "Dangers of research into chronic fatigue syndrome". BMJ. 342: d3780. doi:10.1136/bmj.d3780.
- ↑ Wessely, Simon; Marsh, Stefanie (August 6, 2011). "Interview with Professor Simon Wessely". The Times. Archived from the original on August 6, 2011.
- ↑ "This man faced death threats and abuse. His crime? He suggested that ME was a mental illness". The Sunday Times. May 5, 2013.
- ↑ "It's safer to insult the Prophet Mohammed than to contradict the armed wing of the ME brigade". The Telegraph. September 28, 2012.
- ↑ Tymes Trust (August 2014), Behind the scenes: Setting up the UK CFS/ME Research Collaborative (UK CMRC) (PDF)
- ↑ 174.0 174.1 174.2 Information Commissioner's Office, UK (March 18, 2015), FOI Decision Notice: to QMUL (PDF)
- ↑ 175.0 175.1 175.2 Kings College London (December 11, 2015), FOI Request: Response from Kings College London to James Coyne (PDF), archived from the original (PDF) on December 12, 2015
- ↑ 176.0 176.1 Sheridan, Anna (November 1, 2015), FOI Request: Raw 6min walking test data after treatment
- ↑ 177.0 177.1 McPhee, Graham (July 28, 2015), FOI Request: Fitness data for PACE trial
- ↑ 178.0 178.1 The Lancet (May 17, 2011), "Editorial: Patients' power and PACE", The Lancet, doi:10.1016/S0140-6736(11)60696-X
- ↑ 179.0 179.1 Swan, Norman; Sharpe, Michael; Horton, Richard (April 18, 2011), "Health Report - Comparison of treatments for chronic fatigue syndrome - the PACE trial", ABC Radio National (Australia) - Health Report, archived from the original on April 18, 2011
- ↑ Kennedy, Angela (November 19, 2012). "Statement re Simon Wessely and claims of harassment". Academia.
- ↑ Lewandowsky, Stephan; Bishop, Dorothy (January 25, 2016), "Research integrity: Don't let transparency damage science", Nature News
- ↑ Lewandowsky, Stephan (February 4, 2016), "Most Christians are definitely not terrorists", Shaping Tomorrows World, archived from the original on September 21, 2017
- ↑ Tuller, David (February 1, 2016). "Trial By Error, Continued: A Few Words About "Harassment"". Virology blog.
- ↑ #MEAction (February 1, 2016), Tuller: PACE authors "wrapping themselves in victimhood"
- ↑ Science Media Centre; Crawley, Esther (October 2011). "Threats of Persecution" (PDF). Science Media Centre. p. 16. Archived from the original (PDF) on August 7, 2014.
- ↑ Science Media Centre; Blithell, Claire (February 2013). "Supporting experts targeted by extremists" (PDF). Science Media Centre. p. 10. Archived from the original (PDF) on April 18, 2016.
- ↑ Hale, Catherine (December 17, 2015), "The politics of stigma with ME/CFS", Catherine Hale (blog)
- ↑ @MEMilitant1 (October 8, 2017). "RC Psych previously smeared patients - Will this change?" (Tweet). Archived from the original on March 28, 2018 – via Twitter.
- ↑ Peter Tatchell [@PeterTatchell] (September 21, 2016). "Isaac Marks - Lessons not Learnt" (Tweet) – via Twitter.
- ↑ Peter Tatchell [@PeterTatchell] (September 23, 2016). "Worrying how the DWP treats disabled people" (Tweet) – via Twitter.
- ↑ 191.0 191.1 Eliot Smith, Valerie (January 14, 2019). "Changing the narrative #2: warring factions, divide & rule and death threats". ValerieEliotSmith. Retrieved August 14, 2019.
- ↑ 192.0 192.1 King's College London (December 12, 2012). "Freedom of information act request 138299, response 341512". Whatdotheyknow. Retrieved March 22, 2019.
- ↑ King's College London (January 17, 2017). "Freedom of Information Act request 370916, response 921879" (PDF).
- ↑ D.G. (February 19, 2016). "Reader's comment on Leonid Schneider's blog - PACE team disregarded the MRC data sharing policy". ForBetterScience Blog.
- ↑ Coyne, James (December 4, 2015), "Update on my formal request for release of the PACE trial data", Quick Thoughts blog
- ↑ 196.0 196.1 Coyne, James (February 14, 2016), "A call for the unconditional release of the PLOS One PACE data Part 1", Quick Thoughts blog
- ↑ McCrone, Paul; Sharpe, Michael; Chalder, Trudie; Knapp, Martin; Johnson, Anthony L.; Goldsmith, Kimberley A.; White, Peter D. (August 1, 2012). van Baal, Pieter H.M. (ed.). "Adaptive Pacing, Cognitive Behaviour Therapy, Graded Exercise, and Specialist Medical Care for Chronic Fatigue Syndrome: A Cost-Effectiveness Analysis". PLoS ONE. 7 (8): e40808. doi:10.1371/journal.pone.0040808. ISSN 1932-6203. PMC 3411573. PMID 22870204.
- ↑ Coyne, James (March 7, 2016), "Update: PLoS One affirms my (and anyone else's) right to PACE data published there", Quick Thoughts blog
- ↑ Science Media Centre; Fox, Fiona (October 31, 2013), "SMC - malicious use of FOI against researchers", Science Media Centre
- ↑ (various authors) (September 27, 2015). "Peter White complaining about PACE Trial FOI request and calling for changes to FOI laws". Phoenix Rising.
- ↑ Information Commissioner's Office, UK (March 29, 2016), FOI Decision Notice: to QMUL (PDF)
- ↑ 202.0 202.1 Information Commissioner's Office, UK (October 27, 2015), FOI Decision Notice: to QMUL (PDF)
- ↑ Courtney, Robert (October 26, 2012), FOI Request: PACE Trial Recovery Rates and Positive Outcome Rates
- ↑ Sheridan, Anna (September 3, 2013), FOI Request: 6min walking test data - Recovered patients in PACE trial
- ↑ Peters, John (June 29, 2016), "Using public money to keep publicly funded data from the public", JohnTheJack Blog
- ↑ Anderssen, Alex (July 9, 2016). "QMUL spend £250,000 on PACE data tribunal". The MEAction Network.
- ↑ Eliot Smith, Valerie (July 15, 2016). "Short Update on the Progress of the PACE Trial judgment (QMUL v IC and Matthees)". ValerieEliotSmith Blog.
- ↑ Information Commissioner's Office, UK (August 12, 2016), FOI Decision Notice: to QMUL (PDF)
- ↑ 209.0 209.1 Eliot Smith, Valerie (August 16, 2016). "Tribunal orders release of PACE Trial data (QMUL v the IC and Matthees)". ValerieEliotSmith Blog.
- ↑ 210.0 210.1 Queen Mary University of London (QMUL) (August 16, 2016). "Statement: Disclosure of PACE trial data under the Freedom of Information Act". QMUL.
- ↑ PACE Trial Management Group; (via FOI request) (March 9, 2006), PACE trial protocol: Full Trial Consent Form with Missing Therapist and No Cover (PDF)
- ↑ Coyne, James (November 11, 2015). "Why the scientific community needs the PACE trial data to be released". MindTheBrain Blog.
- ↑ Coyne, James (December 2, 2015). "What it takes for Queen Mary to declare a request for scientific data "vexatious"". Quick Thoughts blog.
- ↑ Coyne, James (December 18, 2015), "King's College London stalls some more, reiterating refusal to release the PACE trial data", Quick Thoughts blog
- ↑ Coyne, James (December 22, 2015). "Recognizing when "protecting patient privacy" is mere excuse for not sharing data". Quick Thoughts blog.
- ↑ Coyne, James (January 2, 2016). "Glimpses into the assault on data sharing". Quick Thoughts blog.
- ↑ Coyne, James (January 31, 2016). "Further insights into war against data sharing: Science Media Centre's letter writing campaign to UK Parliament". Quick Thoughts blog.
- ↑ Coyne, James (February 20, 2016), "As major medical journals balk, BMJ moves forward with routine data sharing", Quick Thoughts blog
- ↑ Coyne, James (March 9, 2016), "UK expert: AIDS data should not be shared until requesters shown to be HIV", Quick Thoughts blog
- ↑ Coyne, James (March 26, 2016), "'We don't share data.' Why Peter White's Wall Street Journal letter can be ignored", Quick Thoughts blog
- ↑ Davis, Ronald W; Levin, Bruce; Racaniello, Vincent R; Tuller, David (December 17, 2015), "Open letter: A request for data from the PACE trial", Virology blog
- ↑ Tuller, David (January 19, 2016). "At least we're not vexatious". Virology blog.
- ↑ Anderssen, Alex (May 14, 2016), "Racianello: PACE Obfuscation will continue "until we are all dead"", The MEAction Network
- ↑ Emerge Australia (March 20, 2016), Open Letter to QMUL - Request for the release of PACE trial data
- ↑ ME/CFS Australia (South Australia) (March 30, 2016), Open Letter to QMUL - Request for the release of PACE trial data
- ↑ Phoenix Rising (March 7, 2016), Open Letter to QMUL - Request for the release of PACE trial data
- ↑ Action for ME (February 12, 2016). "Open Letter to QMUL - Request for the release of PACE trial data".
- ↑ Invest in ME (IIME) (February 12, 2016), Open Letter to The Lancet - Request for the release of PACE trial data
- ↑ Invest in ME (IIME) (February 19, 2016), Open Letter to MRC - Request for the release of PACE trial data
- ↑ ME Association (February 9, 2016). "Open Letter to QMUL - Request for the release of PACE trial data".
- ↑ The Young ME Sufferers Trust (Tymes Trust) (February 16, 2016). "Open Letter to QMUL - Request for the release of PACE trial data".
- ↑ ME Research UK (February 18, 2016), Open Letter to QMUL - Request for the release of PACE trial data
- ↑ Hope for ME and Fibro Northern Ireland (February 16, 2016), Open Letter to QMUL - Request for the release of PACE trial data
- ↑ Welsh Association of ME & CFS Support (WAMES) (February 17, 2016), Open Letter to QMUL - Request for the release of PACE trial data
- ↑ 25% ME Group (February 10, 2016), Open Letter to QMUL - Request for the release of PACE trial data
- ↑ Irish ME Trust (February 22, 2016), Open Letter to QMUL - Request for the release of PACE trial data (PDF)
- ↑ National ME/FM Action Network (Canada) (February 14, 2016), Open Letter to The Lancet - Request for the release of PACE trial data
- ↑ Wake Up Call Beweging (WUCB); ME-gids (March 7, 2016), Open Letter to QMUL - Request for the release of PACE trial data
- ↑ Groep ME Den-Haag (February 24, 2016), Open Letter to QMUL - Request for the release of PACE trial data
- ↑ ME/cvs Vereniging; ME/CVS Stichting Nederland; Steungroep ME en Arbeidsongeschiktheid (March 15, 2016), Open Letter to QMUL - Request for the release of PACE trial data (PDF)
- ↑ Smith, Richard (December 16, 2015), "QMUL and King's college should release data from the PACE trial", BMJ Blogs
- ↑ Smith, Richard; Roberts, Ian (April 29, 2016), "Time for sharing data to become routine: the seven excuses for not doing so are all invalid", F1000Research, 2016 (5): 781, doi:10.12688/f1000research.8422.1
- ↑ Coyne, James (August 18, 2016), "Release the PACE trial data: My submission to the UK Tribunal", Quick Thoughts blog
- ↑ Ellis, Clark (January 25, 2016), "PACE trial's forbidden fruit - is the data really poisonous?", AutoDidact Blog
- ↑ Ellis, Clark (August 29, 2016), "PACE and the interests of trial participants", AutoDidact Blog
- ↑ ME Association (March 16, 2016), PACE Trial data release : Our March website poll gathers 1,322 votes so far
- ↑ Dockser Marcus, Amy (March 7, 2016). "Patients, Scientists Fight Over Research-Data Access". Wall Street Journal.
- ↑ ME Association (March 7, 2016). "Patients, Scientists Fight Over Research-Data Access (Wall Street Journal)".
- ↑ Kennedy, Brian; Stephenson, Darryl; Watson, Nigel. "First-tier tribunal: Information Rights Appeal EA/2015/0269" (PDF). p. 40.
The evidence of [expert witness] Professor Anderson that third parties could not identify participants from the information alone and that, when pressed, he said that the chance of an "activist" being able to discover information that would lead to individual identification was remote, it was clear that his assessment of activist behaviour was, in our view, grossly exaggerated and the only actual evidence was that an individual at a seminar had heckled Professor Chalder. The identity of those questioning the research, who had signed an open letter or supported it, was impressive.
- ↑ Eliot Smith, Valerie (November 26, 2015). "Queen Mary University of London to appeal Information Commissioner's decision on disclosure of PACE Trial data". ValerieEliotSmith Blog.
- ↑ QMUL (November 23, 2015). "Notice of Appeal" (PDF). ValerieEliotSmith Blog.
- ↑ Eliot Smith, Valerie (April 2016). "Mr Alem Mathees Main response to Tribunal as Second Respondent" (PDF). ValerieEliotSmith Blog.
- ↑ "Appeal Number EA/2015/0269 Queen Mary University of London vs The Information Commissioner and Alem Matthees | First-Tier Tribunal | Information Rights" (PDF). Information Commissioner's Office. August 16, 2016. Archived (PDF) from the original on 2016.
- ↑ Anderssen, Alex (August 16, 2016). "Tribunal orders release of PACE data". The MEAction Network.
- ↑ "QMUL spend £250,000 on PACE data tribunal". The MEAction Network. July 9, 2016. Retrieved October 6, 2010.
- ↑ Peters, John (September 14, 2016). "Using public money to keep publicly funded data from the public". Johnthejack. Retrieved October 6, 2022.
- ↑ "A Plea for Decency to White, Chalder & Sharpe". The MEAction Network. September 21, 2016. Retrieved October 6, 2022.
- ↑ "Statement: Disclosure of PACE trial data under the Freedom of Information Act". Queen Mary University Of London. September 9, 2016. Archived from the original on September 10, 2016. Retrieved September 10, 2016.
- ↑ James Coyne [@CoyneoftheRealm] (July 29, 2017). "Whoa, sorry, here is the link to the #PACE data 4 all you budding research parasites. Get analyzing. Expose the lies" (Tweet). Retrieved October 6, 2022 – via Twitter.
- ↑ 260.0 260.1 Wilshire, C; Kindlon, T; Matthees, A; McGrath, S (2017). "Can patients with chronic fatigue syndrome really recover after graded exercise or cognitive behavioural therapy? A critical commentary and preliminary re-analysis of the PACE trial". Fatigue: Biomedicine, Health & Behavior. 5 (1): 43–56. doi:10.1080/21641846.2017.1259724.
- ↑ Centre for Welfare Reform (UK) (August 19, 2016), Major breakthrough on PACE trial, Centre for Welfare Reform
- ↑ "The PACE Trial: Where "Recovery" Doesn't Mean Getting Your Health Back". MEAction. December 14, 2016. Retrieved October 6, 2022.
- ↑ Wilshire, Carolyn; Kindlon, T; McGrath, S (2017). "PACE trial claims of recovery are not justified by the data: a rejoinder to Sharpe, Chalder, Johnson, Goldsmith and White". Fatigue: Biomedicine, Health & Behavior. 5 (1): 62–67. doi:10.1080/21641846.2017.1259724.
- ↑ "PACE Data Sharing Policy" (PDF). Queen Mary University Of London. Archived from the original (PDF) on February 7, 2019.
- ↑ Coyne, James (March 18, 2017), "Don't bother to apply: PACE investigators issue guidance for researchers requesting access to data", Quick Thoughts blog
- ↑ "Special issue: The PACE Trial". Journal of Health Psychology. 22 (9). August 2017.
- ↑ David F Marks [@david_f_marks] (July 29, 2017). "The #PACEtrial team - the only authors to request no peer review. A few red faces on Monday - but will it be anger or shame?" (Tweet). Retrieved October 6, 2022 – via Twitter.
- ↑ "The making of the PACE scandal". Citizen Network. Centre for Welfare Reform. July 28, 2017. Retrieved October 6, 2022.
- ↑ Coyne, James (July 30, 2017). "Last ditch attempt to block publication of special issue of Journal of Health Psychology foiled". Retrieved October 6, 2022.
- ↑ Coyne, James (August 1, 2017). "Part 2: What to look for in a Special Issue of Journal of Health Psychology concerning the PACE trial". Quick Thoughts blog.
- ↑ 271.0 271.1 "Expert reaction to Journal of Health Psychology's Special Issue on The PACE Trial". Science Media Centre. January 31, 2013. Archived from the original on July 31, 2017.
- ↑ Tuller, David (August 2, 2017). "Trial by Error: The Science Media Centre's Desperate Efforts to Defend PACE". Virology blog.
- ↑ John Peters [@johnthejack] (August 8, 2016). "According to a whistleblower, the person who complained to Sage prior to publication of the JHP special issue was Prof M Sharpe #PACEtrial" (Tweet). Retrieved October 6, 2022 – via Twitter.
- ↑ Whipple, Tom; Moody, Oliver (August 1, 2017). "Scientists trade insults over myalgic encephalomyelitis (ME) study". The Times. Retrieved October 6, 2022.
- ↑ Whipple, Tom; Moody, Oliver (August 1, 2017). "A battle of prescriptions". The Times. Retrieved October 6, 2022.
- ↑ "The Times: Scientists trade insults over myalgic encephalomyelitis (ME) study | August 1, 2017". ME Association. August 1, 2017. Retrieved October 6, 2017.
- ↑ "The Times: A battle of prescriptions | 1 August 2017". ME Association. August 1, 2017. Retrieved October 6, 2017.
- ↑ "Is chronic fatigue syndrome real? | Life-threatening condition can leave sufferers bed bound". Daily Express. August 10, 2017. Retrieved October 6, 2017.
- ↑ 279.0 279.1 Burns, Jerome (August 1, 2017). "Angry scientists throw insults at each other over the results of a £5 million taxpayer-funded study into chronic fatigue syndrome". Daily Mail Online. Retrieved October 6, 2022.
- ↑ Rosenbaum, Leah (January 8, 2017). "A New Clue Is Helping to Solve the Mystery Behind Chronic Fatigue Syndrome". Seeker. Retrieved October 6, 2022.
- ↑ Michael VanElzakker [@MBVanElzakker] (August 15, 2017). "Fair-ish. Reminds me of both-sides-ism in climate science. You'd not know from this article that the PACE team are a tiny, isolated faction" (Tweet). Retrieved October 6, 2022 – via Twitter.
- ↑ "'You're a disgusting old fart neoliberal hypocrite' – scientists in furious row over ME study'". iNews. August 1, 2017. Retrieved October 6, 2022.
- ↑ Winter, George (October 16, 2017). "Is it just ME or is this a real disease?". Medical Independent. Archived from the original on October 23, 2017. Retrieved October 6, 2022.
- ↑ Friedberg, Fred (April 2017). "Adverse Impact on Research of the #PACETrial?". IACFS/ME Newsletter. 10 (2) – via TwitLonger.
- ↑ Radford, Giles; Chowdhury, Sonya (2016). "Research Funding | An Overview Of Activity By Major Instutional Funders Included On The Dimensions Database" (PDF). ME Association. Retrieved October 6, 2010.
- ↑ Rehmeyer, Julie (September 21, 2016). "Bad science misled millions with chronic fatigue syndrome. Here's how we fought back". Stat News.
- ↑ 287.0 287.1 Dimmock, Mary; Spotila, Jennie (August 18, 2016). "AHRQ agrees: GET useless, CBT ineffective". Occupy ME.
- ↑ The PLOS ONE Editors (May 2, 2017). "Expression of Concern: Adaptive Pacing, Cognitive Behaviour Therapy, Graded Exercise, and Specialist Medical Care for Chronic Fatigue Syndrome: A Cost-Effectiveness Analysis". PLOS ONE. 12 (5): e0177037. doi:10.1371/journal.pone.0177037. ISSN 1932-6203. PMC 5412692. PMID 28463341.
- ↑ "Data sharing in clinical research: challenges and open opportunities". PLoS. May 2, 2017. Retrieved July 19, 2020.
- ↑ "PLOS upgrades flag on controversial PACE chronic fatigue syndrome trial; authors "surprised"". Retraction watch. May 2, 2017.
- ↑ ME Association (July 11, 2017). "CDC removes CBT and GET as recommended treatments for ME/CFS | 11 July 2017". ME Association.
- ↑ Tuller, David (July 26, 2017). "CDC Removes Reference to Disputed ME/CFS Therapies From Website". Undark.
- ↑ Rehmeyer, Julie; Tuller, David (September 5, 2017). "Why did it take the] CDC so long to reverse course on debunked treatments for chronic fatigue syndrome?". STAT.
- ↑ "For People With Chronic Fatigue Syndrome, More Exercise Isn't Better". NPR. October 2, 2017.
- ↑ Brody, Jane E. (November 12, 2017). "New Recognition for Chronic Fatigue". New York Times.
- ↑ Medical Research Council (August 28, 2018). "Criticism of the PACE trial". mrc.ukri.org. Retrieved April 9, 2019.
- ↑ "The claim that the cure for the crippling fatigue of ME/CFS was to change your mind always seemed bizarre. Now it really is on the way out…". HealthInsightUK. April 22, 2019. Retrieved April 23, 2019.
- ↑ Dimmock, Mary (January 2018). "Clinical Guidance for ME: "Evidence-Based" Guidance Gone Awry" (PDF).
- ↑ "The Failure of Clinical Guidance for People with ME". The MEAction Network. February 5, 2018. Retrieved October 6, 2022.
- ↑ "A song for ME: Blowin' in the Wind". The MEAction Network. April 24, 2018. Retrieved October 6, 2022.
- ↑ Tuller, David (June 19, 2018). "Trial By Error: An Open Letter to The Lancet, Two Years On". Virology blog.
- ↑ Tuller, David (July 10, 2018). "Trial By Error: Yet Another Appeal to The Lancet, With More On Board". Virology blog.
- ↑ 303.0 303.1 "The Times: Call for review of 'flawed' ME research in Lancet letter". The Times. August 21, 2018. Retrieved April 9, 2019.
- ↑ Torjesen, Ingrid (2015), "Tackling fears about exercise is important for ME treatment, analysis indicates", BMJ: 350
- ↑ "Preparing children for starting primary school". August 27, 2018. ISSN 0140-0460. Retrieved April 9, 2019.
- ↑ 306.0 306.1 Wilshire, Carolyn E.; Kindlon, Tom; Courtney, Robert; Matthees, Alem; Tuller, David; Geraghty, Keith; Levin, Bruce (March 22, 2018). "Rethinking the treatment of chronic fatigue syndrome-a reanalysis and evaluation of findings from a recent major trial of graded exercise and CBT". BMC psychology. 6 (1): 6. doi:10.1186/s40359-018-0218-3. ISSN 2050-7283. PMC 5863477. PMID 29562932.
- ↑ Science Media Centre (March 2018). "Factsheet: CFS/ME – The illness and the controversy" (PDF). Science Media Centre.
- ↑ Science Media Centre (March 2018). "Expert reaction to reanalysis of the PACE trial for chronic fatigue syndrome (CFS) treatments". Science Media Centre.
- ↑ "Science media centre CFS/ME factsheet for journalists: the illness and the controversy". Science for ME. 2018.
- ↑ "Before the headlines analysis – Reanalysis of the PACE trial". Daily Mail. March 2018.
- ↑ "Findings Of Chronic Fatigue Study 'Not Reliable'". Huffington Post. March 22, 2018.
- ↑ "ME Association Press Release: Reanalysis of the PACE trial finds impressive claims are 'not statistically reliable' | 22 March 2018". ME Association. March 22, 2018.
- ↑ "The controversy surrounding the 'unreliable' ME PACE trial". May 8, 2018.
- ↑ Topple, Steve (March 22, 2018). "The mainstream medical community just declared war on people living with ME". The Canary.
- ↑ "Chronic Fatigue Treatment Redux: Questioning the Efficacy of CBT and Graded Exercise", NEJM JWatch, April 6, 2018
- ↑ Rethinking the treatment of chronic fatigue syndrome—A reanalysis and evaluation of findings from a recent major trial of graded exercise and CBT, April 26, 2018 – via European Health Journal
- ↑ Sharpe, Michael; Goldsmith, Kim; Chalder, Trudie (March 12, 2019). "The PACE trial of treatments for chronic fatigue syndrome: a response to WILSHIRE et al". BMC Psychology. 7 (1): 15. doi:10.1186/s40359-019-0288-x. ISSN 2050-7283.
- ↑ Wilshire, Carolyn E.; Kindlon, Tom (March 26, 2019). "Response: Sharpe, Goldsmith and Chalder fail to restore confidence in the PACE trial findings". BMC Psychology. 7 (1): 19. doi:10.1186/s40359-019-0296-x. ISSN 2050-7283. PMID 30914065.
- ↑ Winter, George (March 10, 2018), "Chronic fatigue syndrome: the ongoing medical debate", Journal of Healthcare Management, 24 (3)
- ↑ Davenport, Todd E; Stevens, Staci R; VanNess, J Mark; Stevens, Jared; Snell, Christopher R (October 2019). "Checking our blind spots: current status of research evidence summaries in ME/CFS". British Journal of Sports Medicine. 53 (19): 1198–1198. doi:10.1136/bjsports-2018-099553. ISSN 0306-3674.
- ↑ 321.0 321.1 Geraghty, Keith J (November 1, 2016). "'PACE-Gate': When clinical trial evidence meets open data access". Journal of Health Psychology. 22 (9): 1106–1112. doi:10.1177/1359105316675213.
- ↑ Marks, David; Murray, Michael; Estacio, Emee Vida, eds. (2020). "Long-term Conditions: Diabetes and ME/CFS". Health Psychology: Theory, Research and Practice (6 ed.). SAGE. pp. 483–505. ISBN 1529736153.
- ↑ Hughes, Brian Michael (2018). Psychology in Crisis. Macmillan Education UK. ISBN 9781352003017.
- ↑ Myhill, Sarah (2021). "My Complaint to the GMC about the PACE trial authors". Dr Myhill. Archived from the original on April 18, 2021.
- ↑ Robinson, Craig. "I am showing my support for Dr Myhill's complaint to the GMC about the PACE authors". change.org.
- ↑ 326.0 326.1 "Freedom of Information Act 2000 (FOIA) Decision notice | Queen Mary University of London Mile End Road | Reference: FS50687719" (PDF). Information Commissioner's Office. February 20, 2018.
- ↑ Monaghan, Carol (February 6, 2018). "SNP MP secures debate on controversial DWP-funded ME trial".
- ↑ "Glasgow MP Carol Monaghan secures parliamentary examination of controversial ME trial". The Herald. 2018.
- ↑ 329.0 329.1 UK Parliament (February 20, 2018). "PACE trial and its effect on people with ME (transcript)".
- ↑ Invest in ME Research (February 2018). "SNP MP secures debate on controversial DWP-funded ME trial". Invest in ME Research.
- ↑ "Government-funded ME/CFS trial 'one of greatest medical scandals of 21st century' | 20 February 2018". ME Association. February 20, 2018.
- ↑ "Westminster Hall Debate on the PACE trial and its effects on people with ME". The MEAction Network. February 27, 2018. Retrieved October 6, 2022.
- ↑ "ME AWARENESS WEEK 2018 - Early Day Motions". UK Parliament. 2018.
- ↑ White, PD (May 22, 2003). "Trial Registry ISRCTN54285094". BioMed Central.
- ↑ White, PD (2003). "Trial Registry (Archive) ISRCTN54285094". BioMed Central.
- ↑ PACE Trial Management Group (February 1, 2006), PACE Trial Protocol: Final Version 5.0 (PDF)
- ↑ Science Media Centre (February 17, 2011). "Expert Opinion on ME/CFS Study".
- ↑ "Pushing Limits Can Help Chronic Fatigue Patients". Fox news. February 18, 2011.
- ↑ Moore, Thomas (February 18, 2011). "Talking And Exercise Could Cure ME - Study". Sky News. Archived from the original on February 18, 2011.
- ↑ "Study finds therapy and exercise best for ME". The Guardian. February 18, 2011.
- ↑ "Counselling and exercise appear best for ME". Net Doctor. February 17, 2011.
- ↑ "Trial offers hope for ME sufferers". Daily Express. February 18, 2011. Archived from the original on October 21, 2020.
- ↑ "Got ME? Just get out and exercise, say scientists". The Independent. February 18, 2011.
- ↑ Broyd, Nicky (February 17, 2011). "ME/CFS: 'Pacing yourself isn't the answer'". Boots. Archived from the original on September 11, 2015.
- ↑ Lua error: Internal error: The interpreter has terminated with signal "24".
- ↑ Lua error: Internal error: The interpreter has terminated with signal "24".
- ↑ Lua error: Internal error: The interpreter has terminated with signal "24".
- ↑ Lua error: Internal error: The interpreter has terminated with signal "24".
- ↑ Lua error: Internal error: The interpreter has terminated with signal "24".
- ↑ Lua error: Internal error: The interpreter has terminated with signal "24".
- ↑ Lua error: Internal error: The interpreter has terminated with signal "24".
- ↑ Lua error: Internal error: The interpreter has terminated with signal "24".
- ↑ Lua error: Internal error: The interpreter has terminated with signal "24".
- ↑ Lua error: Internal error: The interpreter has terminated with signal "24".
- ↑ Lua error: Internal error: The interpreter has terminated with signal "24".
- ↑ Lua error: Internal error: The interpreter has terminated with signal "24".
- ↑ Lua error: Internal error: The interpreter has terminated with signal "24".
- ↑ Lua error: Internal error: The interpreter has terminated with signal "24".
- ↑ Lua error: Internal error: The interpreter has terminated with signal "24".
- ↑ Lua error: Internal error: The interpreter has terminated with signal "24".
- ↑ Lua error: Internal error: The interpreter has terminated with signal "24".
- ↑ Lua error: Internal error: The interpreter has terminated with signal "24".
- ↑ Lua error: Internal error: The interpreter has terminated with signal "24".
- ↑ Lua error: Internal error: The interpreter has terminated with signal "24".
- ↑ Lua error: Internal error: The interpreter has terminated with signal "24".
- ↑ Lua error: Internal error: The interpreter has terminated with signal "24".
- ↑ Lua error: Internal error: The interpreter has terminated with signal "24".
- ↑ Lua error: Internal error: The interpreter has terminated with signal "24".
- ↑ Lua error: Internal error: The interpreter has terminated with signal "24".
- ↑ Lua error: Internal error: The interpreter has terminated with signal "24".
- ↑ Lua error: Internal error: The interpreter has terminated with signal "24".
- ↑ Lua error: Internal error: The interpreter has terminated with signal "24".
- ↑ Lua error: Internal error: The interpreter has terminated with signal "24".
- ↑ Lua error: Internal error: The interpreter has terminated with signal "24".
- ↑ Lua error: Internal error: The interpreter has terminated with signal "24".
- ↑ Lua error: Internal error: The interpreter has terminated with signal "24".
- ↑ Lua error: Internal error: The interpreter has terminated with signal "24".
- ↑ Lua error: Internal error: The interpreter has terminated with signal "24".
- ↑ Lua error: Internal error: The interpreter has terminated with signal "24".