- This page was created by volunteers like you!
- Help us make it even better. To learn more about contributing to MEpedia, click here.
- Join the movement
- Visit #MEAction to find support or take action. Donate today to help us improve and expand this project.
- Congratulations!
- MEpedia has got over 30 million views as of August 2022!
Mast cell activation syndrome
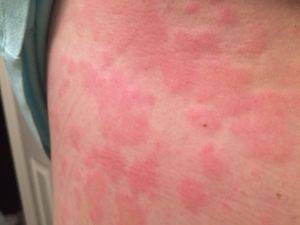
Mast cell activation syndrome (MCAS) is a disorder where mast cells are normal in number, but release excessive amounts of chemicals known as mast cell mediators including histamine. The symptoms of MCAS can be very similar to that of myalgic encephalomyelitis (ME), and therefore may be confused. Moreover, it is also possible to have ME and MCAS simultaneously.
Signs and Symptoms[edit | edit source]
In MCAS, the body's mast cells are being created in normal number, but are over responsive to dietary or environmental triggers. Both of these situations might lead an individual to have excess histamine in circulation. Excess histamine can cause severe inflammation and a wide variety of symptoms. Almost any organ system in the body can be affected by MCAS.[1] Because a variety of symptoms can be present, MCAS is commonly misdiagnosed.
A confounding element in diagnosing MCAS is that signs and symptoms occur in almost all areas of the body. The symptoms might wax and wane.
Most patients experience fatigue, fevers, and sensitivity to individualized environmental "triggers." Other commonly identified signs and symptoms are as follows:
- Hot flashes, irregular heartbeat, high or low blood pressure (hypotension and/or hypertension)
- vertigo, dizziness, forgetfulness, depression or anxiety, headaches, insomnia, restlessness
- hives or other visible skin rashes
- arthritis, muscle pain, bone pain, osteoporosis/osteopenia
- anaphylaxis (a severe allergic reaction)
- Malabsorption and gastrointestinal distress leading to low iron and low Vitamin D and low Vitamin B12[2]
Diagnosis[edit | edit source]
MCAS can be difficult to diagnose as the cause of the syndrome is still considered to be unknown. In 2010, a criteria for diagnosing MCAS was proposed by Dr. Cem Akin and colleagues. These criteria suggest that two or more organ systems must be affected; this can include gastrointestinal, cardiovascular, skin, or respiratory. If given anti-histamine or mast cell therapy, the patient's symptoms must improve. Thirdly, the patient should be tested for serum tryptase, an enzyme secreted by mast cells during the peak of a symptomatic episode. If tryptase is >15ng/mL, the patient may have MCAS. Urine and blood tests should be collected more than once to confirm a positive diagnosis. Prostaglandin and histamine levels can be also be tested.[3]
Clinicians[edit | edit source]
There are a very few mast cell specialists working in the United States. An expert is Dr. Lawrence Afrin formerly at the University of Minnesota now in in Armonk, NY. Drs. Clem Akin and Mariana Castells run a mastocytosis clinic at Brigham and Women's Hospital in Boston, United States. More integrative doctors are beginning to be aware of mast cell activation syndrome, but it remains elusive in both treatment and diagnosis.
Comorbidities[edit | edit source]
Afrin et al. (2016) found the most common comorbities in MCAS, occurring in over 10% of patients, were
- gastroesophageal reflux disease (GERD) 35%
- hypertension 29%
- multiple/atypical drug reactions 23%
- abdominal pain not otherwise specified 22%
- hysterectomy/oophorectomy 21%
- hyperlipidemia 20%
- cholecystectomy 20%
- environmental allergies 19%
- tobacco abuse 18%
- asthma 18%
- diabetes mellitus type 2 17%
- hypothyroidism 17%
- headaches 17%
- depression 16%
- sinusitis 16%
- fibromyalgia 16%
- anemia of chronic inflammation 15%
- sleep apnea 15%
- frequent upper respiratory tract infections 15%
- miscarriage 15%
- pharyngitis (sore throat caused by inflammation) or tonsillitis 14%
- dysmenorrhea 14%
- thromboembolism 13%
- frequent or atypical infections 13%
- obesity 13%
- osteoarthritis 13%
- anxiety/panic 12%
- vertebral disease 12%
- cardiovascular malformations 12%
- dermatitis 11 %
- presyncope or syncope 11 %
- interstitial cystitis 11 %
- chronic kidney disease 10%
- POTS 10%[4]
MCAS is often diagnosed in patients that have been previously diagnosed with Ehlers-Danlos syndrome (EDS), a heritable connective tissue disorder, and with postural orthostatic tachycardia syndrome (POTS), a form of orthostatic intolerance. Both of these conditions are also commonly co-morbid with ME. The overlap between EDS, POTS, and MCAS is thought to be due to increased tryptase production.[5]
An extra copy of the gene TPSAB1 has been noted as a possible cause for increased tryptase production.[6] The TPSAB1 has also been implicated in pruritus, unexplained, gastrointestinal symptoms, and in other diseases including the recently discovered Hereditary Alpha Tryptasemia.[7][8]
A small study by Novak et al. (2022) found decreased cerebral blood flow and small fiber neuropathy were very common in MCAS patients.[9]
Myalgic Encephalomyelitis and Chronic Fatigue Syndrome[edit | edit source]
Most MCAS patients have fatigue, and approximately half have cognitive dysfunction, and many symptoms of ME/CFS are also found; this may result in other a misdiagnosis of chronic fatigue syndrome or be the result of having both illnesses.[4] The rate of post-exertional malaise, which is now considered the hallmark of ME/CFS, is unknown in MCAS patients.
Research on the relationship between mast cells and ME is in its infancy. One study found that individuals diagnosed with moderate to severe ME have been noted to have higher amounts of dysfunctional mast cells in circulation.[10]
At a two-day physician summit in Salt Lake City, Utah in March 2018, physicians discussed the relationship between chronic fatigue syndrome and mast cell activation syndrome.[11]
- Charles Lapp: "I see a lot of this. I think it's one of the many overlap syndromes that we've been missing for years."
- Susan Levine: "I suspect 50% to 60% of ME/CFS patients have it. It's a very new concept."...In Levine's experience, MCAS often manifests in patients being unable to tolerate certain foods or medications. "If we can reduce the mast cell problem, we can facilitate taking other drugs to treat ME/CFS," she said. However, she also cautioned, "It's going to be a subset, not all ME/CFS patients."
Common triggers[edit | edit source]
Mast cell degranulation can be triggered by a wide range of foods absorbed via the alimentary tract; environmental exposures inhaled through the nose such as mold, diesel fuel and chemical fragrances; emotional or physical stress; exercise; direct skin contact with chemicals, and even sunlight. While many lists exist describing the range of possible triggers, many people tolerate triggers others cannot and a person’s list of triggers can be highly individual.[12][1]
Potential treatments[edit | edit source]
Summary[edit | edit source]
| Name | Type | Form | Availability | Mechanism of action | Notes |
|---|---|---|---|---|---|
| Claritin (Loratadine) | H1 Blocker | OTC | Low anticholinergic activity. Not sedating. | ||
| Benadryl | H1 Blocker | Oral and IV | OTC, prescription | Sedating. | |
| Tagament (Cimetidine) | H2 Blocker | ||||
| Pepcid (Famotidine) | H2 Blocker | ||||
| Axid (Nizatidine) | H2 Blocker | ||||
| Zantac (Ranitidine) | H2 Blocker | Oral, injection, IV | Withdrawn from market in US[13] | ||
| Zyrtec | H1 blocker | OTC | Not sedating. | ||
| Allegra (Fexofenadine) | H1 blocker | Not sedating. | |||
| Ketotifen (Zaditor/Zaditen, Zyrtec Itchy Eye Drops) | H1 Blocker, mast cell stabilizer[14][15] | Oral, eye drops.[14][15] | Perscription or OTC.[15][14] | Not sedating. | |
| Quercetin | OTC | ||||
| Diamine oxidase | Enzyme normally produced by the body | Breaks down histamine. Especially found in gut and placenta. | |||
| Omalizumab (Xolair) | Antibody and mast cell stabilizer.[16] | Prescription[17] | |||
| Vitamin C | OTC | Inhibits mast cell production and degranulation; increases diamine oxidase; breaks down histamine. | Sustained release version is recommended[18] | ||
| Magnesium | OTC | Co-factor in diamine oxidase | [19] | ||
| Prednisone | Corticosteroid | Oral, injection | Prescription |
Elimination diet[edit | edit source]
Treatment of MCAS invariably involves trigger identifcation and avoidance[18]. The Royal Prince Alfred Hospital in NSW, Australia has developed an elimination diet that focuses on reducing common food chemical triggers such as salicylates, amines, glutamates and particular additives.
Vitamin C[edit | edit source]
Numerous studies have found Vitamin C to be inversely correlated with histamine and that the administration of Vitamin C reduces blood histamine levels.[20][21][22][23] It does this potentially through several mechanisms: by inhibiting mast cell production; by increasing diamine oxidase (an enzyme that breaks down histamine and is chiefly found in the gut); by inhibiting mast cell degranulation (and the release of histamine in the first place),[24] and by inhibiting histidine decarboxylase (the enzyme that forms histamine).[25]
Magnesium[edit | edit source]
Like Vitamin C, magnesium is a co-factor in the production of diamine oxidase. Magnesium deficiency has been seen to increase mast cell production in some cases; therefore magnesium supplementation may be helpful in controlling mast cell division.[26]
Antihistamines[edit | edit source]
Over-the-counter H1 and H2 antihistamine blockers such as Allegra (Fexofenadine), Zyrtec (Cetirizine), Claritin (Loratadine), and compounded Zaditor/Zaditen (Ketotifen) are common treatments for MCAS.[19][27] It is recommended that the patient should consult a physician for secondary symptom treatment or targeted mast cell therapies.[25]
Some patients also use herbal antihistamine supplements such as quercetin or take diamine oxidase (DAO), an enzyme normally produced by the body that breaks down histamine, as a supplement.
Prescription drug treatments include Xolair (Omalizumab), which has been proposed as a possible mast cell stabilizer and is used in allergic asthma and chronic urticaria.
Leukotriene inhibitors[edit | edit source]
These include Montelukast and Zafirlukast.[19] Montelukast and zafirlukast block the effects of leukotriene C4 (LTC4) and zileuton blocks LTC4 production, so these reduce wheezing and abdominal cramping.[28] Montelukast carries a black box label warning for possibly serious psychiatric adverse effects including psychosis and suicidal ideation.[29]
Mast cell stabilizers[edit | edit source]
These include oral cromolyn sodium (Gastrocrom) and Ketotifen (Zaditor/Zaditen).[19]
Acetylcholinesterase inhibitors[edit | edit source]
In 2015, a large German study found 29% of ME/CFS patients had elevated autoantibodies to M3 and M4 muscarinic acetylcholine receptors, as well as ß2 adrenergic receptors.[30][31] A 2016 Australian study found that ME/CFS patients had significantly greater numbers of single nucleotide polymorphisms associated with the gene encoding for M3 muscarinic acetylcholine receptors.[32] One study found that acetylcholine via muscarinic receptors strongly inhibited the release of histamine in mucosal mast cells.[33] Mestinon, an acetylcholinesterase inhibitor, and vagus nerve stimulators can increase the amount of acetylcholine circulating in the peripheral nervous system.
Omalizumab[edit | edit source]
Omalizumab is an anti-IgE monoclonal antibody approved for treating other allergic diseases. In a study of 55 French patients with mast cell disorders, 43 patients achieved a "best response" and 76.7% of the responding patients achieved a persistent response (three months or longer.)[34] Median time to first response was 2 months and median time to best response was 6 months. One severe adverse event occurred, with researchers suggesting this recommends initiating treatment in hospital, but otherwise found the safety profile acceptable.[34]
Sauna[edit | edit source]
There is some limited evidence that sauna may be useful in antihistamine resistant urticaria, an allergic skin condition that involves mast cell activation and the production of excess histamine.[35]
Notable studies[edit | edit source]
- 2016, Characterization of Mast Cell Activation Syndrome[4] - (Full text)
- 2011, Mast cell activation disease: a concise practical guide for diagnostic workup and therapeutic options. Journal of Hematology & Oncology[2] - (Full text)
- 2020, Diagnosis of mast cell activation syndrome: a global "consensus-2"[36] - (Full text)
- 2019, Doctor, I Think I Am Suffering from MCAS: Differential Diagnosis and Separating Facts from Fiction[37] - (Full text)
Learn more[edit | edit source]
- MCAS: Treatment - Mast Attack
See also[edit | edit source]
References[edit | edit source]
- ↑ Jump up to: 1.0 1.1 Akin, Cem (August 2017). "Mast Cell Activation Syndromes". The Journal of Allergy and Clinical Immunology. 140 (2): 349–355.
- ↑ Jump up to: 2.0 2.1 Molderings, Gerhard J; Brettner, Stefan; Homann, Jürgen; Afrin, Lawrence B (March 22, 2011). "Mast cell activation disease: a concise practical guide for diagnostic workup and therapeutic options". Journal of Hematology & Oncology. 4: 10. doi:10.1186/1756-8722-4-10. ISSN 1756-8722. PMC 3069946. PMID 21418662.
- ↑ Akin, Cem (2010). "Mast Cell Activation Syndrome: Proposed Diagnostic Criteria". J Allergy and Clinical Immuno. 126 (6): 1099-104.e4. doi:10.1016/j.jaci.2010.08.035.
- ↑ Jump up to: 4.0 4.1 4.2 https://doi.org/10.1182/blood.V128.22.3683.3683
- ↑ Milner, Joshua (2015), Research Update: POTS, EDS, MCAS Genetics, Washington DC: Dysautonomia International Conference & CME
- ↑ Lyons, Jonathan J. (August 1, 2018). "Hereditary Alpha Tryptasemia: Genotyping and Associated Clinical Features". Immunology and Allergy Clinics of North America. Mastocytosis. 38 (3): 483–495. doi:10.1016/j.iac.2018.04.003. ISSN 0889-8561. PMC 6411063. PMID 30007465.
- ↑ Cheung, Ingrid; Vadas, Peter (February 2015). "A New Disease Cluster: Mast Cell Activation Syndrome, Postural Orthostatic Tachycardia Syndrome, and Ehlers-Danlos Syndrome". The Journal of Allergy and Clinical Immunology. 135 (2): Supp AB65.
- ↑ "Hereditary Alpha Tryptasemia Syndrome FAQ". National Institute of Allergy and Infectious Diseases. October 17, 2016.
- ↑ Novak, Peter; Giannetti, Matthew P.; Weller, Emily; Hamilton, Matthew J.; Castells, Mariana (March 1, 2022). "Mast cell disorders are associated with decreased cerebral blood flow and small fiber neuropathy". Annals of Allergy, Asthma & Immunology. 128 (3): 299–306.e1. doi:10.1016/j.anai.2021.10.006. ISSN 1081-1206.
- ↑ Nguyen, T.; Johnston, S.; Chacko, A.; Gibson, D.; Cepon, J.; Smith, D.; Staines, D.; Marshall-Gradisnik, S. (2017). "Novel characterisation of mast cell phenotypes from peripheral blood mononuclear cells in chronic fatigue syndrome/myalgic encephalomyelitis patients". Asian Pac J Allergy Immunol. 35 (2): 75-81. doi:10.12932/AP0771.
- ↑ Tucker, Miriam IE. (March 13, 2018). "Mast Cell Activation May Underlie 'Chronic Fatigue Syndrome'". Medscape. Retrieved September 25, 2018.
- ↑ Frieri, Marianne (2015). "Mast Cell Activation Syndrome". Allergy and Immunology.
- ↑ "Zantac". drugs.com. Retrieved February 16, 2021.
- ↑ Jump up to: 14.0 14.1 14.2 "Ketotifen Monograph for Professionals". Drugs.com. Retrieved April 5, 2022.
- ↑ Jump up to: 15.0 15.1 15.2 "Ketotifen (Oral)". Drugs.com. October 26, 2021. Retrieved April 5, 2022.
- ↑ Kumar, Calvin; Zito, Patrick M. (February 20, 2022). Omalizumab. Treasure Island (FL): StatPearls Publishing. Retrieved April 5, 2022.
- ↑ "Omalizumab". drugs.com. February 20, 2022. Retrieved April 5, 2022.
- ↑ Jump up to: 18.0 18.1 Weinstock, Leonard B.; Pace, Laura A.; Rezaie, Ali; Afrin, Lawrence B.; Molderings, Gerhard J. (April 2021). "Mast Cell Activation Syndrome: A Primer for the Gastroenterologist". Digestive Diseases and Sciences. 66 (4): 965–982. doi:10.1007/s10620-020-06264-9. ISSN 0163-2116.
- ↑ Jump up to: 19.0 19.1 19.2 19.3 "Medications to Treat Mast Cell Diseases". The Mast Cell Disease Society. Retrieved February 16, 2021.
- ↑ Clemetson, C.A. (April 1980). "Histamine and ascorbic acid in human blood". The Journal of Nutrition. 110 (4): 662–668. ISSN 0022-3166. PMID 7365537.
- ↑ Johnston, C.S.; Martin, L.J.; Cai, X. (April 1992). "Antihistamine effect of supplemental ascorbic acid and neutrophil chemotaxis". Journal of the American College of Nutrition. 11 (2): 172–176. ISSN 0731-5724. PMID 1578094.
- ↑ Johnston, CS (December 1996). "Vitamin C depletion is associated with alterations in blood histamine and plasma free carnitine in adults". J Am Coll Nutr.
- ↑ Yazdani, Shaik (2016). "Relationship Between Vitamin C, Mast Cells, and Inflammation". J Nutr Food Sci.
- ↑ Mio, M (1999). "Ultraviolet B (UVB) light-induced histamine release from rat peritoneal mast cells and its augmentation by certain phenothiazine compounds". Immunopharmacology.
- ↑ Jump up to: 25.0 25.1 Molderings, G (July 2016). "Pharmacological treatment options for mast cell activation disease". Naunyn Schmiedebergs Arch Pharmacol. 389 (7): 671–94. doi:10.1007/s00210-016-1247-1. PMC 4903110. PMID 27132234.
- ↑ Takemoto, S (2013). "Magnesium deficiency induces the emergence of mast cells in the liver of rats". J Nutr Sci Vitaminol. 59 (6): 560–3. doi:10.3177/jnsv.59.560.
- ↑ Klimas, Lisa (October 27, 2014). "MCAS: Treatment". Mast Attack. Retrieved December 8, 2018.
- ↑ "Mast Cell Activation Syndrome (MCAS)". www.aaaai.org. Retrieved April 11, 2023.
- ↑ "Montelukast: MedlinePlus Drug Information". medlineplus.gov. Retrieved April 11, 2023.
- ↑ Loebel, Madlen; Grabowski, Patricia; Heidecke, Harald; Bauer, Sandra; Hanitsch, Leif G.; Wittke, Kirsten; Meisel, Christian; Reinke, Petra; Volk, Hans-Dieter (February 2016). "Antibodies to β adrenergic and muscarinic cholinergic receptors in patients with Chronic Fatigue Syndrome". Brain, Behavior, and Immunity. 52: 32–39. doi:10.1016/j.bbi.2015.09.013. ISSN 1090-2139. PMID 26399744.
- ↑ "Autoantibodies found in subset of CFS patients". The MEAction Network. Retrieved August 10, 2018.
- ↑ Marshall-Gradisnik, Sonya; Smith, Peter; Nilius, Bernd; Staines, Donald R. (January 1, 2015). "Examination of Single Nucleotide Polymorphisms in Acetylcholine Receptors in Chronic Fatigue Syndrome Patients". Immunology and Immunogenetics Insights. 7: III.S25105. doi:10.4137/III.S25105. ISSN 1178-6345.
- ↑ "Acetylcholine via Muscarinic Receptors Inhibits Histamine Release from Human Isolated Bronchi". American Journal of Respiratory and Critical Care Medicine. doi:10.1164/ajrccm.156.2.96-12079#.v7vo-zmrlmv.
- ↑ Jump up to: 34.0 34.1 "A new therapy to calm down mast cells". American Academy of Allergy, Asthma & Immunology. April 9, 2019. Retrieved July 28, 2019.
- ↑ Magen, Eli (2014). "Beneficial Effect of Sauna Therapy on Severe Antihistamine-Resistant Chronic Urticaria" (PDF). Israeli Medical Association Journal.
- ↑ Afrin, Lawrence B.; Ackerley, Mary B.; Bluestein, Linda S.; Brewer, Joseph H.; Brook, Jill B.; Buchanan, Ariana D.; Cuni, Jill R.; Davey, William P.; Dempsey, Tania T.; Dorff, Shanda R.; Dubravec, Martin S. (May 1, 2021). "Diagnosis of mast cell activation syndrome: a global "consensus-2"". Diagnosis. 8 (2): 137–152. doi:10.1515/dx-2020-0005. ISSN 2194-802X.
- ↑ Valent, Peter; Akin, Cem (April 1, 2019). "Doctor, I Think I Am Suffering from MCAS: Differential Diagnosis and Separating Facts from Fiction". The Journal of Allergy and Clinical Immunology: In Practice. 7 (4): 1109–1114. doi:10.1016/j.jaip.2018.11.045. ISSN 2213-2198. PMID 30961836.

