- This page was created by volunteers like you!
- Help us make it even better. To learn more about contributing to MEpedia, click here.
- Join the movement
- Visit #MEAction to find support or take action. Donate today to help us improve and expand this project.
- Congratulations!
- MEpedia has got over 30 million views as of August 2022!
Post-exertional malaise

In ME/CFS, Post-exertional malaise (PEM) refers to a worsening of the illness after physical or cognitive exertion which was previously tolerated.[2][3][4] This can include a worsening of ME/CFS symptoms, the appearance of new symptoms, and a worsening of “baseline” (capacity for exertion).[5][4] These characteristics are often delayed 24-72 hours or more after the triggering event.[1][6][7][8] PEM is considered to be the hallmark symptom of ME/CFS,[1][9] and interferes with the ability to lead a "normal" life.[3][8][9]
While in most fatiguing diseases patients experience symptom relief after exercise,[10][11] the opposite is true for ME/CFS patients for whom even minimal exertion may cause PEM.[12][13][14] When in PEM, people with ME/CFS have a lower capacity for exertion and the baseline for triggering more PEM is lower.[5]
In ME/CFS recovery time from exertion is prolonged,[15] lasting days, weeks, months, or longer.[16] PEM can lead to a permanent worsening of the condition and increased disability.[17][18][19] Some people with ME refer to these post-exertional episodes as "crashes".[20][21]
Characteristics[edit | edit source]

Image: MEpedia. Data from: Jason (2018). DePaul Symptom Questionnaire: Post-Exertional Malaise short form (DSQ-PEM)[22].
The distinctive characteristics of post-exertional malaise were found by scientific research including patient interviews and questionnaires, then confirmed and expanded on by biomedical research, especially using exercise tests.[22][23][24]
Too much exertion causes ME/CFS patients abnormalities in cognitive functioning,[25][26] immune activation,[27] gene expression[28][29][30][31] and endogenous pain inhibition[32][33][34] in ME/CFS patients that were not seen before exertion or in healthy controls.
Most importantly PEM can be demonstrated by a 2-day cardiopulmonary exercise test (CPET) procedure.[35][21] On the second day CPET, ME/CFS patients display a significant drop in VO2 max and maximal workload, that is not seen in healthy controls or other diseases.[36][37][38][39][40] These objective measures track strongly with the presence, severity and duration of PEM.[41][42] [43] Brian Vastag won a groundbreaking long term disability (LTD) claim using CPET to prove his PEM was a severe disabling symptom.[44][45]
A 2015 review of the literature by the National Academy of Medicine concluded there to be "sufficient evidence that PEM is a primary feature that helps distinguish ME/CFS from other conditions."[41] Disagreement exists however on the precise nature of PEM and how it should be defined,[46] with some diagnostic criteria emphasizing muscle weakness and others a more a general form of fatigue and exhaustion.[47]
The Centers for Disease Control and Prevention (CDC) outline different types of exertion that may trigger PEM and how it impacts patients noting some may be housebound or completely bedbound during a crash. "People with ME/CFS may not be able to predict what will cause a crash or how long it will last."[3]
Causes[edit | edit source]
PEM can be caused by mental/cognitive as well as physical exertion[2] and the symptom complex it invokes does not necessarily relate to the initial trigger.[1] ME/CFS patients suffer from a post-exertional flu-like feeling,[48] with brain fog,[15] photophobia and other symptoms not usually reported after exertion.[1] In contrast to most forms of exercise intolerance, the onset of PEM is frequently delayed[49] with many patients reporting the height of their symptom flare-up, two[15] or several days after the initial trigger.
Examples[edit | edit source]
Examples of PEM given by the CDC are:
- attending a child's school event may leave a patient housebound for a couple of days unable to do needed tasks, like laundry; grocery shopping may cause a crash that requires a nap in the car before driving home or a call for a ride home;
- a shower may leave a patient bedbound and unable to do anything for days;
- keeping up with work may lead to spending evenings and weekends recovering.[3]
PEM described by Dr. David Kaufman[edit | edit source]
Diagnosis and Management of Myalgic Encephalomyelitis and Chronic Fatigue Syndrome By Dr. David Kaufman/Unrest
(Video begins @2:16 and PEM is described until @3:35. View entire 11:47 for a full description of ME/CFS)
This video on the diagnosis and management of myalgic encephalomyelitis and chronic fatigue syndrome is part of the Unrest Continuing Education module. US medical providers can register to watch Unrest online for free and receive Continuing Education credit.[21]
Patients' description[edit | edit source]







An illness within an illness[edit | edit source]
PEM refers to a worsening of many ME/CFS symptoms as a result of physical or mental exertion. It consists of more than post-exertional fatigue and pain, and can cause severe debility.[5] As one patient described it:
"When I do any activity that goes beyond what I can do—I literally collapse—my body is in major pain, it hurts to lay in bed, it hurts to think, I can’t hardly talk—I can't find the words, I feel my insides are at war."[41]
Another patient emphasized that the feeling of PEM is very different from what one experiences as a healthy person:
"PEM is like nothing else you will experience in healthy life; a combination of a hangover, the flu, finishing a 10k run, all at the same time at varying levels of severity."[57]
Considering the serious but fluctuating debility PEM causes, ME/CFS expert Dr. Anthony Komaroff described it as "an illness within an illness".[58]
Energy conservation and pacing[edit | edit source]
Patients often report the feeling of a red line, an energy limit that if exceeded, will result in a crash or relapse. As one [[Norway|Norwegian] patient described:
“....And suddenly it is just too much. The body turns itself off, as if it has gone on strike. You have pushed too much for too long, it repeats itself, and the body stops functioning.”[59]
Energy conservation strategies such as pacing and the envelope theory have been developed to minimize PEM while allowing patients to stay as active as possible.[60] These techniques advise patients to balance energy availability and expenditure and to recognize early signs of PEM so they can reduce activity levels before a relapse occurs.
The distinctive characteristics of PEM[edit | edit source]
Four aspects differentiate the post-exertional malaise of ME/CFS patients from the exercise intolerance commonly reported in patients suffering from deconditioning or other conditions.
Timing[edit | edit source]
First of all, there is the time lapse. While physical complaints are usually reported during or shortly after exercise, PEM often has a delayed onset, hours or sometimes even days after the original trigger. Yoshiuchi et al. (2007) for example wrote that: "after a briefer maximal exercise task, reports of worsening CFS symptoms were inconsistent or absent until 5 days after the challenge, a pattern not typically observed in real life."[49] The authors noted that this delay could be used to distinguish ME/CFS from other fatiguing illness. Another study from Stanford University showed that in up to 37% of the 150 ME/CFS patients studied, PEM may not begin until a day or more after an exertional trigger.[1]

Patients may not be familiar with this characteristic of their relapses, since it is very counter-intuitive. As one patient noted:
"It's really counter-intuitive to feel bad after a delay of 24 hours after exertion. It may take quite some time before people even make that connection, if ever. I only noticed it about three years in, and I hesitated to mention to others because I thought it might make me sound nuts."[61]
Another time-related characteristic of PEM is a prolonged recovery period. In a 2010 study 25 M/CFS patients and 23 matched controls were followed up for seven days after performing a maximal cardiopulmonary exercise test. After two days, all controls subjects were recovered while only one ME/CFS patient was. Most (60%) of the ME/CFS participants reported that it took more than five days to fully recover from the test and many reported feeling at their worst 24 to 48 hours after the test.[15] Other studies have found the same prolonged recovery period in ME/CFS patients after exertion. A Dutch study for example noted:
"For CFS patients, daily observed fatigue was increased up to 2 days after the exercise test. For controls, self-observed fatigue returned to baseline after 2 h."[62]
Lapp et al. followed 31 ME/CFS patients for 12 days after performing a maximal exercise test of 8-10 minutes. The average relapse lasted 8.82 days, although 22% of patients were still in relapse when the study ended at 12 days.[63] In the Stanford study by Chu et al. 87% of respondents indicated that they endure PEM for 24 hours or more. The authors concluded:
"In many medical conditions, exertion-exacerbated symptoms usually start during exertion or immediately after and usually resolve immediately or shortly after exertion stops. In contrast, PEM may not start until hours or even days after the trigger starts or has been removed, may peak after the first day, and may not stop until hours to months later. This characteristic of PEM often leads patients and clinicians to believe that symptom exacerbations are random rather than associated with a trigger; most people will not intuit that symptoms are caused by a trigger that occurred hours to days prior unless specifically asked by their clinicians to pay attention."[1]
Type of symptoms[edit | edit source]
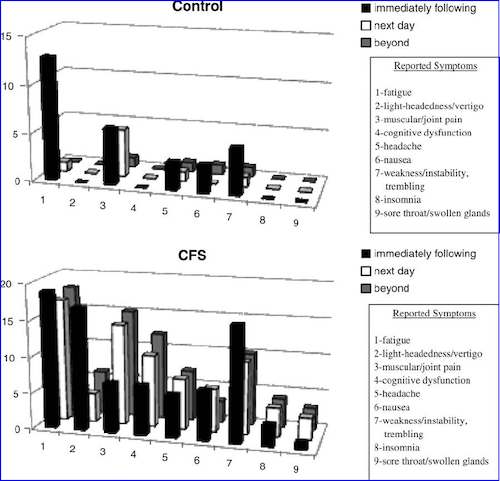
The second characteristic of PEM is the type of symptoms reported. The Canadian Consensus Criteria, a 2003 clinical guideline formed by experts in the field, underlines that many PEM symptoms are immune-related:
"The malaise that follows exertion is difficult to describe but is often reported to be similar to the generalized pain, discomfort and fatigue associated with the acute phase of influenza. Delayed malaise and fatigue may be associated with signs of immune activation such as a sore throat, lymph glandular tenderness and/or swelling, general malaise, increased pain or cognitive fog."[48]
VanNess et al. noted how cognitive difficulties after exertion differentiate ME/CFS patients from healthy controls:
"Another interesting difference between groups was the reported symptom of cognitive dysfunction, for example, brain-fog or difficulty concentrating. Problems of this nature were not reported by any of the control subjects, whereas 12 patients (48%) experienced these problems: "Carrying on conversations was hard." "Can't think straight." "My mind was not clear."[15]
This was elaborated by Chu et al., the research team who conducted the first in-depth investigation on how ME/CFS patients describe their PEM:
"There exists no medical condition the authors are familiar with where exertion or emotional distress causes immune/ inflammatory-related symptoms like sore throat, tender lymph nodes, or flu-like feelings, yet 60% and 36% of our subjects, respectively, reported these symptoms with either stimuli and about a quarter experienced all 3 with exertion. Conversely, symptoms typically associated with physical exertion in other conditions, like shortness of breath or chest pain in chronic lung or heart disease, are rarely reported in ME/CFS. Furthermore, it is well-established that physical activity improves mood, sleep, and pain in both healthy people as well those with chronic illnesses like depression or anxiety yet our subjects report worsened sleep, mood, and pain with physical activity."[1]
Triggers[edit | edit source]
A third characteristic of PEM is that it can be elicited by multiple types of triggers. Research has shown that ME/CFS patients experience PEM after cognitive effort, physical or emotions.[5] A 2014 study for example followed up on 32 ME/CFS patients after completing a battery of neurocognitive tests. As the authors concluded: "following a challenging cognitive demand, fatigue significantly increased two days after testing," which was "suggestive of post-exertional symptom exacerbation following mental effort."[2] Commenting on the outbreak in West Kilbride, Ayrshire, Ramsay remarked:
"Once the disease was established the most characteristic symptom was extreme exhaustion, particularly after exercise. The exhaustion also occurred after emotional or mental strain."[64]
Some other precipitants of PEM that have been reported include positional changes and exposure to excessive light or sounds[65]. While PEM was often thought of as symptom exacerbation after exercise, it is clear that for some ME/CFS patients even basic activities of daily living such as toileting, bathing, dressing, communicating, and reading can trigger relapses.[66] As long time ME/CFS expert Jennifer Spotila explained in a four-piece exploration of the phenomenon post-exertional malaise:
"The use of the word ‘exertion’ may create the impression that PEM is triggered by strenuous or intense activity, but this is not the case […] Some patients need only attempt to make a simple meal or get dressed before PEM descends."[67]
This was confirmed by Chu et al.
"[…] our results provide formal evidence supporting patient narratives, clinician experiences, and current case definitions which assert that even tasks like walking, cooking, or reading can provoke PEM."[1]
In some instances, the specific trigger of PEM cannot be identified.[66]
Loss of functional capacity[edit | edit source]
A fourth distinctive element of PEM is often described as a loss of stamina and/or functional capacity. This refers to the results of the 2-day cardiopulmonary exercise test (CPET) procedure. A CPET is usually reproducible and normally has a test-retest difference of 7-12%[35]. ME/CFS patients however show strikingly lower results on several measures at the second CPET compared to the first, despite meeting objective markers of maximal effort. These results have been replicated by several research teams, though there is inconsistency on which measure (VO2 or maximal workload, at peak or ventilatory threshold), the decline in functional capacity is best represented.
| Physiological changes between first and second exercise test during 2-day CPET procedure in patients with ME/CFS | |||||||
|---|---|---|---|---|---|---|---|
| Number of ME/CFS patients | VO2 peak | VO2 at VT | Workload peak | Workload at VT | HR peak | O2pulse at VT | |
| VanNess et al. 2007.[39] | 6 | -22% | -26% | ? | ? | ? | ? |
| Vermeulen et al. 2010.[37] | 15 | -6.3% | -7.0% | -5.3% | -7.0% | -1.9% | -8.8% |
| Snell et al. 2013.[36] | 51 | -5% | -10.8% | -7.2% | -55.2% | ? | ? |
| Keller et al. 2014.[38] | 22 | -13.8% | -15.8% | -12.5% | -21.3% | -5.9% | -12.6% |
| Hodges et al. 2018.[40] | 10 | +5.3% | +6.1% | -6.7% | -11.4% | -0.6% | ? |
The drop in functional capacity on the second CPET is usually not seen in other diseases. According to Keller et al. (2014) "ME/CFS patients currently represent a unique class of ill patients who do not reproduce maximal CPET measures, unlike individuals with cardiovascular disease, lung disease, end-stage renal disease pulmonary arterial hypertension and cystic fibrosis".[38] A preliminary study from New Zealand suggests that patients with MS do not display the same decline on the second day of exercise testing, as do patients with ME/CFS.[40]
Questions have however been raised about the clinical use of the 2-day CPET procedure. Snell et al. (2013) suggested it might be unethical to use this method since many ME/CFS patients might suffer a serious relapse as a result of exercise performance.[36] Others have noted that the CPET- procedure is not practical either. It cannot be used in patients with severe ME/CFS (thus excluding these patients from study) and because of cost and expertise, may not be available to most clinicians.[66] CPET for ME/CFS is usually not covered by insurance and can cost hundreds of dollars.[22] For these reasons PEM is usually assessed using self-reporting questionnaires.
PEM in children[edit | edit source]
It is important to understand that in children with ME/CFS may not describe having PEM. They can experience a "crash" or relapse from exertion, perhaps from just taking the school bus, having to spend prolonged periods in bed.[68]
Differentiation[edit | edit source]
Several studies have shown that PEM is the symptom of ME/CFS that best differentiates it from other diseases.
Healthy controls and idiopathic chronic fatigue[edit | edit source]
PEM was one of the symptoms in the CDC symptom inventory list that differentiated subjects with ME/CFS from those with long term chronic fatigue without ME/CFS.[69] It was also the highest loading factor among a data set of 38 measurements used for a principal component analysis of unexplained chronic fatigue.[70] Data for this study came from the epidemiological study in Wichita, Kansas.
The other major epidemiological study, carried out in Chicago, also identified PEM as the hallmark symptom of ME/CFS. In a 10 year follow-up study on the 32 patients originally identified as having ME/CFS, all of the contacted patients reported post-exertional malaise at some point in time. This symptom was able to differentiate ME/CFS patients with those with idiopathic chronic fatigue, those with exclusionary illnesses and healthy controls. According to the author:
"Among all the variables in this study, only for post-exertional malaise did the CFS group significantly differ from the other three conditions. This reaffirms the importance of this being a cardinal and critical symptom for CFS."[71]
Using a large sample of ME/CFS patients from Newcastle, Norway and the Solve_ME/CFS_Initiative#Biobank Solve ME/CFS Biobank, Jason et al. (2014) conducted an analysis of different case definitions and symptoms. The domain of post-exertional malaise was found to be most adequate at differentiating ME/CFS patients from controls. As the authors noted:
Using the latent variables from the empiric criteria, only one factor (PEM) was needed to reach a sensitivity of 90.8%, specificity of 92.5% and accuracy of 91.6%, and this was the only data mining where all percentages were over 90%. […] the fact that PEM came out in all analyses supports the importance of this domain in the case definition.[72]
A 2014 examination, using 236 patients and 86 controls, showed that three symptoms accurately classified 95.4% of participants as patient or control: fatigue/extreme tiredness, inability to focus on multiple things simultaneously, and experiencing a dead/heavy feeling after starting to exercise.[73] Another data mining study by the same research group, suggested the selection of four symptoms: next to extreme tiredness, unrefreshing sleep and difficulty finding the right word to say or expressing thoughts, PEM was once again represented with the item “physically drained/sick after mild activity.”[74]
Maes et al. (2012) divided ME/CFS patients into two groups: those with or without PEM lasting for more than 24 hours. Analysis showed this to be a meaningful division as the former group (45% of the sample) not only had higher symptom scores on concentration difficulties and a subjective experience of infection, but also higher markers of immune-activation such as IL-1, TNFα, lysozyme and neopterin, than the CFS group without PEM. According to the authors their findings, "underscore the relevance of post-exertional malaise to identify a subgroup of CFS patients that should be diagnosed as ME".[75]
Multiple sclerosis[edit | edit source]
According to a 2015 report by the National Academy of Medicine, the prevalence of PEM among ME/CFS patients varies from 69 to 100%, which is much higher than in other disease groups.[41] In a 1996 study by Komaroff et al. 13 of 25 MS-patients (52%) reported PEM[76], a figure similar to what Jason et al. found with the DSQ PEM subscale in a cohort of 106 MS-patients.[77] Both studies used a broad definition of PEM which focused on fatigue after exercise. Preliminary research suggests that adding more specific questions, for example about the prolonged recovery and various type of triggers, PEM might be able to differentiate ME/CFS from multiple sclerosis. A 2018 study for example showed that ME/CFS patients reported to experience PEM more often through mental exertion and to recover more slowly from PEM compared to multiple sclerosis patients.[22]
Major Depressive disorder[edit | edit source]
In the 1996 study by Komaroff et al. (2006) only 19% of patients with major depression reported PEM.[76] A similar figure was found by Hawk et al., who found PEM in 3 patients in their sample of 15 with major depressive disorder.[78] In contrast all of the 15 studied ME/CFS patients reported PEM, making it the largest discriminant function for all investigated symptoms. White et al. studied patients with persistent symptoms of fatigue and poor concentration after glandular fever. According to the authors "the complaint of post-exertional physical fatigue may help to differentiate post-viral fatigue states from psychiatric disorders."[79]
Gulf war illness[edit | edit source]
Baraniuk and Shivapurkar (2017) looked at MicroRNAs (miRNA) in the cerebrospinal fluid of ME/CFS patients, healthy controls and patients with Gulf War Illness before and after an exercise challenge (a submaximal bicycle exercise). While there were no differences in miRNA between the groups at baseline, a distinct signature appeared after exercise. According to the authors, "exercise caused distinct patterns of miRNA changes in CFS and […] GWI indicating significant pathophysiological differences between conditions."[80] A 2013 study under the guidance of Nancy Klimas compared the immune signature in 30 Gulf war patients, 22 ME/CFS patients and 30 controls, after an graded exercise test. Results indicated the importance of physical exercise for differentiating these different groups:
"Common to both GWI and CFS illness signatures were the direct or indirect contributions of IL-10 and IL-23 expression though these occurred at very different times. While levels measured at rest supported an illness signature in GWI, their impact in CFS was only observable during and after exercise, again emphasizing the importance of a challenge and response timeline in distinguishing these illnesses."[81]
A study by Washington et al. (2020) found different brain activation patterns after exercise in patients with Gulf War Illness compared to ME/CFS, including the opposite response in some areas, despite both illnesses causing post-exertional malaise.[82] The same study found that brain activation patterns did not change after exercise in healthy controls.[82]
Objective findings after exertion:[edit | edit source]
In the 1980s Melvin A. Ramsay stressed the use of assessing ME patients after exertion. Regarding muscle weakness – what he regarded as the hallmark symptom of the disease –he noted:
"If muscle power is found to be satisfactory, a re-examination should be made after exercise; a walk of half a mile is sufficient, as very few ME case can manage more. […] It is most important to stress the fact that cases of ME of mild or even moderate severity may have normal muscle power in a remission. In such cases, test for muscle power should be repeated after exercise."[64]
Though the definition of PEM has been expended far beyond muscle weakness, modern day research has confirmed the utility of testing ME/CFS after exertion. Many markers that are normal in resting state in ME/CFS patients turn out to be abnormal after a physical or cognitive stressor.[41]
A fairly small study of ME/CFS patients who met the widely used Fukuda criteria found different brain activation patterns in particular areas of the brain in ME/CFS patients after exercise compared to before exercise; these changes in brain activation was not found in healthy controls after exercise.[82]
Gene expression[edit | edit source]
One example is gene expression. In a 2009 study Light et al. showed that after a moderate exercise test, the leukocytes of ME/CFS patients showed an increase in expression of adrenergic, metabolite detecting and immune-related genes that was not seen in healthy controls. Before the exercise test there were no abnormalities in the expression of these genes of ME/CFS patients. The authors speculated this to be evidence for sensitization of fatigue pathways in ME/CFS.[28] The research team was able to confirm their results in a subsequent study using a larger sample of 48 patients.[29] In a 2012 comparison MS patients also displayed an increase in post-exercise gene expression, but only ME/CFS patients showed increases in metabolite-detecting sensory receptors. According to the authors:
"Because only the CFS patients showed increases in these metabolite-detecting receptors, the sensory receptor elements of this gene profile seem particularly specific to CFS and may reflect dysregulated pathways that directly contribute to increased effort sense during exercise and postexertional malaise."[31]
Attempts at replication by other research teams have produced contradictory results. Meyer et al. were unable to confirm most of the post-exertional increases in gene expression, except for some in the adrenergic and glucocorticoid pathway.[30] An Australian team under the guidance of Andrew Lloyd failed to find any significant exercise-induced changes in leucocyte gene expression, though the patient sample used (n = 10) was rather small and did not include any patients with severe disability.[83]
Immune activation[edit | edit source]
There are many studies demonstrating exercise-induced immunological abnormalities in ME/CFS patients.[27] Most findings however still have to be replicated by other research groups, using larger samples.
Oxidative stress[edit | edit source]
In 2005 the French team Jammes et al. found a lengthened and accentuated oxidative stress response in ME/CFS patients after a cycling exercise until exhaustion. At baseline markers of oxidative stress (thiobarbituric acidreactiv substances and ascorbic acid) did not differ significantly from healthy controls. After the exercise challenge however, the oxidative stress response occurred sooner and lasted longer in the ME/CFS group. This was associated with alterations in muscle excitability (lengthened M-wave duration) in ME/CFS-patients, which were not seen in controls.[84] A small 2009 follow-up study confirmed these results and associated it with a post-exertional reduction of heat shock proteins HSP 27 and HSP 70 after exercise.[85] According to the authors, this is another indication of an impaired redox status in ME/CFS patients. A 2011 study confirmed most of these results in a larger cohort of 43 ME/CFS patients and 23 healthy controls. Again the data indicated an increased exercise-induced oxidative stress and a reduced Hsp response. Though it is known that deconditioning can increase oxidative stress, the authors argued this to be unlikely in their study population, for several reasons:
“…deconditioning can be ruled out in our study because (i) it induces carbohydrate and lipid disorders that were not observed during routine biochemical check-up in these CFS patients, (ii) CFS patients did not have reduced maximal exercise performance or an accentuated lactic acid response and (iii) we found no correlation between the duration of CFS symptoms […] and the resting levels of oxidant–antioxidant status and HSPs.”[86]
A Canadian research team had already reported a marked decline of HSP 27 during the post-exercise period of six ME/CFS patients in 2002.[87]
Complement C4a [edit | edit source]
In 2003 Sorensen et al. found that the complement split product C4a was increased after exercise in the 20 ME/CFS patients, but not in controls. Furthermore a significant correlation was found between the increase in C4a and total symptom score.[88] C4a is generated from the cleavage of the native complement protein C4 via the classical and lectin pathways. A follow up study, published in 2009, found that other elements of the lectin pathway also responded differently to an exercise challenge in ME/CFS patients compared to controls. Both C4 and mannan-binding lectin serine protease 2 (MASP2) were observed at higher levels in ME/CFS subjects 1 hour post-exercise.[89] The authors speculated this to contribute to the increased C4a split product 6 hours after the exercise challenge. In a 2010 study by Nijs et al. there was no increase in C4a after exercise in ME/CFS patients, though a significant correlation with post-exertional pain and fatigue was found.[90]
Cytokines[edit | edit source]
The expression of cytokines after physical exercise has been researched in ME/CFS patients since the mid-1990s. Most of these studies have found negative results (see table below).
| Study | Number of participants | Exercise challenge | Cytokines tested: | Results: |
| Peterson et al. (1994)[91] | 10 (Holmes criteria, all cases were post-infectious) | Walking 1 mile per hour for 30 min | IL-1 β, IL-6, and TNF-α, TGF-β | Negative results |
| Lloyd et al. (1994)[92] | 12 (Australian criteria) | 30 min hand grip exercises | IFN-γ, IFN-α, IL-1 β, TNF-α | Negative results |
| La Manca et al. (1999)[93] | 20 (Fukuda criteria) "only patients with an illness duration of less than 6 years, who reported at least substantial intensity on symptom severity scales in the month prior to recruitment and who had no major psychiatric diagnosis in the 5 years prior to illness onset" were included | An exhaustive treadmill exercise test | IL-2, IL-4, IL-10, IFN-γ, TNF-α | Negative results |
| Cannon et al. (1997)[94] | 8 (Holmes criteria) “their chronic illness began abruptly with a "flu-like" condition, (c) they had been ill for less than 3 years, and (d) they regularly experienced postexertional malaise” | Stepping up and down on a platform for 15 min | IL-1 β, interleukin 1 receptor antagonist (IL-1Ra), and soluble interleukin 1 receptor type II (IL-1sRII). | Negative results |
| Gupta et al. (1998)[95] | 5 (Holmes criteria) | 30 min hand grip exercises | IL-6 | Negative results |
| Cannon et al. (1999)[96] | 10 (Holmes criteria) their chronic illness began abruptly with a "flu-like" condition, (c) they had been ill for less than 3 years, and (d) they regularly experienced postexertional malaise” | Stepping up and down on a platform for 15 min | IL-1 β, IL-6 | Negative results |
| Jammes et al. (2009)[85] | 9 (Fukuda criteria) 6/9 had practiced sport at high level, for more than 4 years before the symptoms occurred. | Cycling test until maximal work load | IL-6, TNF-a | Negative results |
| Robinson et al (2010)[97] | 6 (Fukuda criteria) | Incremental exercise test to exhaustion | IL-6, sIL-6R and sgp130 | Negative results |
| Andrea White et al. (2010)[98] | 19 (Fukuda criteria) | The authors used "a moderate whole-body exercise task (working both arms and legs) for 25 min that was mild enough that all CFS patients were able to complete it successfully but did induce a flare of fatigue and pain symptoms that remained above pre-exercise levels for 48 h post-exercise in the majority of patients." | IL-1β, IL-2, IL-12, TNF-α, soluble CD40L, IFN-γ, IL-4, IL-10, IL-13, IL-6 and IL-8
|
Positive results for a subgroup (11/19) of patients with high PEM
|
| Andrew Lloyd et al. (2018)[99] | 24 (Fukuda criteria) "we used the 1994 Centers for Disease Control (CDC)/Fukuda international diagnostic criteria for ME/CFS, but required participants to have post exertional malaise. Terefore, in labeling our patients this refers to the revised international consensus criteria from 2011" | Symptom limited exercise on an ergocycle. | Growth factors: FGF-β, HGF, NGF, PDGF-BB, TGFα, TGF-β1, VEGF
Colony stimulating factors and stem cell factors: G-CSF, GM-CSF, M-CSF, SCF
Interleukins: IL-1α, IL-1β, IL-1RA, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12p40, IL-12p70, IL-13, IL-15, IL-17, IL-17F, IL-18 and LIF
Chemokines: CCL2 (MCP-1), CCL3 (MIP-1α), CCL4 (MIP-1β), CCL5 (RANTES) (RANTES) CCL7 (MCP-3), CXCL1 (Gro-α), CXCL5 (ENA78), CXCL9 (MIG), CXCL10 (IP-10), CCL11 (Eotaxin)
Interferons: INF-α, INF-β, INF-γ
Adhesion Molecules : ICAM-1, VCAM-1
Other factors: CD40L, FASL, leptin, PAI-1, resistin, TNF-α, TNF-β, TRAIL |
Positive results: ME/CFS had a distinct cytokine profile post-exercise. |
Moneghetti et al. took a different approach and looked at the cytokine profiling after exercise, as this may differentiate patients with ME/CFS from sedentary controls. Of the 51 cytokines and growth factors tested, 10 significantly changed after exercise in both groups, a further 7 only changed in controls and five only changed in ME/CFS (namely, CXCL10, IL-8, CCL4, TNF-β and ICAM-1). This suggests a distinct cytokine inflammatory signature in ME/CFS.[99] White et al. (2010) differentiated their 19 ME/CFS patients with a high or low post-exertional malaise (called symptom flare in the study). While the cytokine expression after exercise of patients with low PEM was similar to those of healthy controls, patients with high PEM showed opposite results. As the authors noted:
"In sum, low SF [symptom flare] patients and controls showed a pattern of post-exercise decreases in both pro and anti-inflammatory cytokines (with the exception of increases in IL-8), whereas the high SF [symptom flare] patients showed a pattern of increases in both cytokine types at 8h and no decreases at any time."[98]
Autonomic response[edit | edit source]
Several research teams have noted post-exertional abnormalities in the autonomic function of ME/CFS patients, though the exact meaning of these results is not yet clear.
A Canadian team under the guidance of Terrence Montague noted that during a maximal exercise test, ME/CFS patients have a lower maximal heart rate than controls. The authors noted that:
"...patients with chronic fatigue syndrome have normal resting cardiac function but a markedly abbreviated exercise capacity characterized by slow acceleration of heart rate and fatigue of exercising muscles long before peak heart rate is achieved."[100]
A significantly lower peak heart rate has been repeatedly observed in CPET-studies with ME/CFS patients.[101][102][103] In one of the largest of these into exercise performance, the authors noted the same phenomenon as Montague et al.
“The resting heart rate of the patient group was higher, but the maximal heart rate at exhaustion was lower, relative to the control subjects.”[104]
The Belgium team Van Oosterwijck et al. (2015) reported an impaired heart rate recovery in 20 female ME/CFS patients following exercise.[105] In other disease groups this is associated with risk for cardiac events and sudden death. Cordero et al. (1986) did not find a significant difference in mean heart rate between 11 ME/CFS patients and six healthy controls after walking on a treadmill, but they did find patients to have significantly less 'vagal power', a measure for respiratory-related parasympathetic contributions to heart rate.[106] Soetekouw et al. (1999) noted that during a handgrip exercise, the hemodynamics response was lower in the ME/CFS group than in the control group, although this could be attributed to the lower level of muscle exertion in the ME/CFS group.[107] LaManca et al. studied 19 ME/CFS (Holmes criteria) and found that they had a diminished heart rate and blood pressure in response to a cognitive test compared to healthy controls, though exercise did not magnify this effect.[108] Similar results were found by a Norwegian research group. They studied 13 adolescents with ME/CFS and 53 age-matched controls after a mental stress test (arithmetic questions). Though heart rate was significantly higher in patients at baseline, there were no meaningful differences during the arithmetic challenge.[109] Finally, Ocon et al. (2012) studied 16 patients with both the diagnosis of ME/CFS and POTS after increased orthostatic stress and a cognitive challenge. An impairment of the neurocognitive abilities was noted, that was not seen in healthy controls.[110]
Sleep[edit | edit source]
A first study into the effects of exercise on sleep in ME/CFS found a beneficial effect: approximately half the patients slept better after exercise.[111] A follow-up study by the same research team (under the guidance of Benjamin Natelson) found more post-exercise improvement (transitions to deeper sleep stages) of sleep in ME/CFS patients than in controls. The patients, however, reported more fatigue in the morning after exercise while healthy controls showed significant improvement in sleepiness and fatigue. The authors speculated this to be due to a disruption of the REM sleep: ME/CFS showed, both at baseline and post-exercise, an increased rate of transition from REM to wake compared to controls and this correlated with symptoms of fatigue, pain and sleepiness.[112] An Australian study followed up on 35 ME/CFS patients after performing a physical (stationary cycling) or cognitive (stimulated driving) challenge. While patients spent a greater proportion of wakeful hours lying down, they did not report significant changes in sleep quality or sleep duration. The authors did however note that the expected increase in heart rate variability (HRV) between wake and sleep, was significantly reduced in ME/CFS patients after completing the challenges. These changes in HRV have been associated with the falling asleep, and might be related to the unfreshed sleep of ME/CFS patients.[113] Finally, Ohashi et al. (2002) recorded physical activity for 6-days in 10 patients with ME/CFS and 6 controls before and after performing a maximal treadmill test. Their results indicate an increase in circadian rest-activity in ME/CFS patients after exercise as the activity pattern of patients shifted toward later hours in the day.[114]
Cognitive performance[edit | edit source]
While some studies have found a decreased cognitive performance after exercise in ME/CFS, others have not (see table below).[41] This difference may be due to heterogeneity of the patient sample and methods used.
| Study | Number of ME/CFS subjects | Neurocognitive tests | Results |
| Sonya Marshall et al. (1997)[115] | 8 | Buschke Selective Reminding Test, Continuous-Performance Test-Identical Pairs Version (CPTIP), Paced Auditory Serial Addition Task (PASAT), Stroop Color Word Test, Reaction-Time Tests, Salthouse Reading Span Task (SRST), Verbal Scholastic Aptitude Test (SAT). | Negative |
| Blackwood et al. (1998)[25] | 10 | "The following aspects of cognitive function were examined (in order): working memory/auditory attention (digit span, from WAIS-R); psychomotor speed (digit symbol, also from WAIS-R); word fluency (FAS test, using the letters F and S only); and selective attention and sustained attention (telephone search and lottery tasks respectively, both from the test of everyday attention)” | Positive |
| La Manca et al. (1998)[116] | 19 | The Stroop Color and Word Test, the Symbol Digit Modalities Test (SDMT), an oral version of the Trail Making Test (TMT) and the Serial 13s Test (STT) | Positive |
| Claypoole et al. (2001)[117] | 21 | The Wechsler Adult Intelligence Scale–Revised, Digit Span Forward and Backward subtests, The Hopkins Verbal Learning Test, The Digit Vigilance Test, the Lafayette Clinic Repeatable Neuropsychological Test Battery, Controlled Oral Word Association Test (COWAT) | Negative |
| Cook et al. (2005)[118] | 20 ME/CFS only and 19 ME/CFS with comorbid fibromyalgia | Participants completed cognitive testing using the automated neuropsychological assessment matrices (ANAM) | Negative |
| Yoshiuchi et al. (2007)[49] | 9 | A one-back memory task | Negative |
| Cook et al. (2017)[26] | 15 | The Paced Auditory Serial Addition Task (PASAT) and a simple number recognition task | Positive |
Pain modulation[edit | edit source]
Another post-exertional abnormality reported in ME/CFS is pain modulation. When healthy people exercise, their brain produces endorphins that increase pain thresholds. In some chronic pain patients like fibromyalgia and whiplash associated disorders, this endogenous pain inhibition response is defect and pain thresholds decrease shortly after exercise (i.e. they experience more pain while they should be feeling less). In 2004 Whiteside et al. first showed this defect in ME/CFS patients.[32] These results were confirmed by two studies by the Belgium pain in motion team: while pain thresholds increased in normal controls they decreased in the ME/CFS patient group.[33][34] As a caveat, one must note that these studies only included ME/CFS patients that were suffering from chronic pain, while comorbid FM was not assessed. So it remains unclear if these results will also show up in ME/CFS patients that do not have comorbid FM.[119]
Other[edit | edit source]
The gut microbiome[edit | edit source]
Shukla et al. (2015) found post-exertional changes in the gut microbiome in ME/CFS patients that were not seen in healthy controls. Increased clearance of bacteria in the blood was also noted, which made the authors speculate that exercise induced a bacterial translocation in ME/CFS patients.[120]
Catecholaminergic hyporeactivity[edit | edit source]
Strahler et al. found that ME/CFS patients showed an attenuated response (lower increases) of epinephrine to an exercise challenge, compared to heathy controls. This ‘catecholaminergic hyporeactivity’ was however subtle and short-lived.[121]
Nitric oxide metabolites[edit | edit source]
A Spanish research team found much higher increases of nitric oxide metabolites (nitrates) after a maximal exercise test in 44 ME/CFS patients compared to 25 healthy controls while there were no differences between the groups at baseline.[122]
Problems in defining PEM[edit | edit source]
Asking the right questions[edit | edit source]
Jason et al. (1999) reported that in a group of ME/CFS patients, the percentage endorsing PEM ranged from 40,6 to 93,8% depending on how the question assessed this symptom.[123] The report of the National Academy of Medicine noted that “the prevalence of PEM among ME/CFS patients as diagnosed by existing criteria varies from 69 to 100 percent.”[41]
Some patients try to reduce post-exertional relapses by pacing themselves and reducing exertion that exceeds their energy limits. Questionnaires assessing PEM by frequency instead of propensity, might erroneously label these patients as not having PEM. In a 2015 study, Jason et al. measured ME/CFS patients’ responses to the PEM-criterion in the Fukuda (1994) definition: ‘Do you feel generally worse than usual or fatigued for 24 hours or more after you have exercised?’ Although the majority (75%) endorsed this item, a notable percentage (25%) did not. Yet when the question was framed differently, leaving out the 24 hours’ time period and substituting exercise with normal daily activity, these participants also agreed they experienced high levels of fatigue after normal daily activity.[46] This clearly shows that patients who have already modified their activities to avoid or reduce PEM may potentially show up as false negatives.
Another issue is the definition of PEM in the Fukuda criteria. While the wording used here is vague, the time criterion is rather strict requiring PEM to last more than 24 hours.[124] Some patients do not endorse this item because they only have post-exertional malaise for less than 24 hours.[125] A 2018 study concluded that setting the criterion at 24 hours would exclude almost 30% of ME/CFS patients. It advises that this definition might be useful in research settings but that in a clinical context, a 14-23 hour time period might be more appropriate.[22]
These observations point to the need of a more precise definition of PEM and several attempts to this end have been made.
More than just fatigue and pain[edit | edit source]
Few instruments have assessed PEM adequately. The CDC symptom inventory for example, only asks about fatigue after exertion, while PEM entails much more than that. An Australian group at the University of New South Wales tried to better define PEM, using 19 ME/CFS patients after exposure to different stressors.[126] Participants indicated that the term fatigue did not adequately describe the sensation they experienced on a daily basis. A word frequency analysis of descriptors nominated by these patients indicated 5 themes:
- Exhausted or tired.
- Heaviness in the limbs or whole-body.
- Fogginess in the head.
- Weakness in the muscles.
- Drained of energy.
The DePaul Symptom Questionnaire (DSQ) subscale[edit | edit source]
The instrument most commonly used to assess PEM is a subscale from the DePaul Symptom Questionnaire (DSQ). The DSQ is a 54-item questionnaire was developed in 2010 to operationalize the Canadian Consensus Criteria, providing concrete directives to assess ME/CFS-symptoms with their frequency and severity.[127] In a Norwegian comparison with physician assessments, The DSQ scored a sensitivity of 92% and a specificity of 75%.[128] This indicated that the DSQ is a useful tool in detecting and screening symptoms, but that a follow-up medical examination is necessarily to confirm the diagnosis and identify possible exclusionary medical and psychiatric disorders.
The post-exertional malaise subscale on the DSQ (DSQ-PEM) particularly demonstrated excellent clinical utility as it was able to differentiate between ME/CFS patients and controls.[129] In early 2018 the Common Data Elements working group on PEM formed by NINDS and the CDC, recommended the use of five items from the DSQ to measure PEM.[12]
- Dead, heavy feeling after starting to exercise.
- Next day soreness after non-strenuous, everyday activities.
- Mentally tired after the slightest effort.
- Minimum exercise makes physically tired.
- Physically drained or sick after mild activity.
To meet criteria for post-exertional malaise, one of these items need to be endorsed at sufficient frequency and severity (2 or greater on a scale of 0-4).[22]
Although the DSQ has good test-retest reliability and is regarded as a useful tool in making the diagnosis of ME/CFS, its ability to capture PEM accurately has been questioned. Originally these five items formed one of the five subdomains of the ME/CFS Fatigue Types Questionnaire (MFTQ)[130][131] and critics argue that these items are focused too much on fatigue/tiredness to be an adequate measure of PEM. A document formulated by the Science for ME PEM working group to address these issues, explained:
"The DSQ PEM items focus largely on feeling fatigue or tiredness, and, apart from one item, do not mention that post-exertional symptoms may be delayed. There is no mention of prolonged recovery or the loss of functional capacity."[61]
The NINDS/CDC common data elements PEM subgroup also noted about the DSQ:
"...the instrument does not assess the full range of symptoms that could be exacerbated by PEM and only one item addresses the sometimes delayed onset/ prolonged duration of PEM.[132]
In an online poll to which 783 people responded, 68% answered that the DSQ PEM did not reflect their experience of post-exertional malaise[61], though questions have been raised about the neutrality of the wording used.[131] In response Jason et al. noted that the DSQ PEM items were developed and selected to screen for the presence of PEM, rather than to comprehensively measure all aspects and variations of PEM. A 2018 analysis, using a large patient sample (n = 704), showed that screening items from the DSQ PEM subscale, were able to identify 97% of patients, which was higher than any other item to describe PEM.[131]
Furthermore, the authors later revised the DSQ PEM subscale to include new items, some based on Melvin Ramsay's writings.[22] An extra 5 questions can be used after the initial screening with the DSQ PEM subscale, to better differentiate ME/CFS from other, comparable conditions:
- Do you experience a worsening of your fatigue/energy related illness after engaging in minimal physical effort?
- Do you experience a worsening of your fatigue/energy related illness after engaging in mental effort?
- If you feel worse after activities, how long does this last?
- If you were to become exhausted after actively participating in extracurricular activities, sports, or outings with friends, would you recover within an hour or two after the activity ended?
- If you do not exercise, is it because exercise makes your symptoms worse?
An analysis showed that these questions (the duration of PEM in particular) helped to differentiate ME/CFS patients from controls with MS or post-polio syndrome.[22]
The DePaul Post-Exertional Malaise Questionnaire[edit | edit source]
The DePaul Post-Exertional Malaise Questionnaire (DPEMQ) is a questionnaire based on input from hundreds of patients.[8]
Post-exertional malaise, or a variation of this term, is a key symptom of myalgic encephalomyelitis and chronic fatigue syndrome, as this symptom is mentioned in almost all myalgic encephalomyelitis and chronic fatigue syndrome case definitions. Until now there has not been a comprehensive questionnaire to assess post-exertional malaise. To rectify this situation, in this article we describe the development of a new questionnaire, called the DePaul Post-Exertional Malaise Questionnaire, which was based on input from hundreds of patients. Preliminary validation was provided by the findings of significant and predictable relationships between different domains of this post-exertional malaise questionnaire and physical functioning.[8]
PENE[edit | edit source]
Of all case definitions, the 2011 International Consensus Criteria (ICC)[42] offered the most precise and elaborated definition of the post-exertional symptoms that characterize ME. To differentiate it from post-exertional malaise, the term used in the Fukuda criteria, the authors introduced a new name: Post-Exertional Neuroimmune Exhaustion (PENE). PENE is described as “a pathological inability to produce sufficient energy on demand with prominent symptoms primarily in the neuroimmune regions”[42] and has the following characteristics:
- Marked, rapid physical and/or cognitive fatigability in response to exertion, which may be minimal such as activities of daily living or simple mental tasks, can be debilitating and cause a relapse.
- Post-exertional symptom exacerbation: e.g. acute flu-like symptoms, pain and worsening of other symptoms.
- Post-exertional exhaustion may occur immediately after activity or be delayed by hours or days.
- Recovery period is prolonged, usually taking 24 hours or longer. A relapse can last days, weeks or longer.
- Low threshold of physical and mental fatigability (lack of stamina) results in a substantial reduction in pre-illness activity level.
The definition fails however to make clear how frequent these symptoms must occur to diagnose PENE, but all must be present.
Muscle weakness[edit | edit source]
A more prominent criticism of PENE came from a 2016 factor analysis of PEM, using a large sample of 704 participants. Results suggested that “PEM is composed of two empirically different experiences, one for generalized fatigue and one for muscle-specific fatigue.”[47] The latter refers to the description of ME by Ramsay, where post-exertional muscle weakness was highlighted. This element of PEM was confirmed in a study by the Workwell Foundation where the symptoms of 25 ME/CFS patients and 23 age-matched controls were followed up. As the report noted:
"The two groups also differed with respect to the experience of physical weakness or instability immediately after testing. This was reported by 16 patients (64%) as opposed to 5 controls (22%). Weakness persisted into the next day in 10 patients (40%) but in only 1 control (4%). However, distinct differences can be observed in the severity of the weakness between groups when analyzing their reports. The sole report of weakness from a control stated: '[I had] tired legs when going up stairs—fine overall.' In contrast, statements from CFS patients included: 'Unable to walk without assistance.' '[I experienced] falling from muscle weakness.'[15]
A Norwegian in depth-report of ME/CFS-patients relationship to exercise also highlighted muscle weakness:
"Some related how they would struggle to get home after exercise – one had to stop her car on her way from the fitness centre. Another was walking in the woods and suddenly felt it would be impossible to make his way back home. They described feeling that something completely wrong had happened to their bodies, without understanding what was going on. Thought processes did not work as usual, motor abilities were reduced, or the legs would not move them as they would usually expect. Some participants described a paralyzed feeling subsequent to activity, where a lot of energy would be needed to be able to move."[133]
While many descriptions of PEM like the DSQ subscale assess this element indirectly by asking patients about a dead heavy feeling after exercise or next day soreness, it is fully lacking in the ICC definition of PENE.[47]
Common data elements PEM working group[edit | edit source]
The NINDS/CDC Common Data Elements (CDE) PEM working group emphasized the need of a better definition of PEM. Its draft recommendations highlighted that
"The definition of PEM is based primarily on clinician experience, patient reports and a few formal studies. There is a dearth of studies asking participants about their experiences of PEM in an openended manner, which is needed."[12]
A 2018 analysis showed that patients' preferences to describe PEM are generally not well-represented within present case definition criteria or descriptions.[131] Although the CDE working group acknowledged the need to device a better instrument to assess PEM, it currently promotes the use of the DSQ PEM subscale as a screening tool, after which a clinician's assessment is advised to diagnose PEM. The CDE PEM working group also provided a description of PEM, based on the 2015 literature review by the National Academy of Medicine:
"PEM is defined as an abnormal response to minimal amounts of physical or cognitive exertion that is characterized by:
- Exacerbation of some or all of an individual study participant's ME/CFS symptoms. Symptoms exacerbated can include physical fatigue, cognitive fatigue, problems thinking (e.g. slowed information processing speed, memory, concentration), unrefreshing sleep, muscle pain, joint pain, headaches, weakness/instability, light-headedness, flu-like symptoms, sore throat, nausea, and other symptoms. Study participants can experience new or non-typical symptoms as well as exacerbation of their more typical symptoms.
- Loss of stamina and/or functional capacity.
- An onset that can be immediate or delayed after the exertional stimulus by hours, days or even longer.
- A prolonged, unpredictable recovery period that may last days, weeks, or even months.
- Severity and duration of symptoms that is often out-of-proportion to the type, intensity, frequency, and/or duration of the exertion. For some study participants, even basic activities of daily living like toileting, bathing, dressing, communicating, and reading can trigger PEM."[12]
Symptom recognition[edit | edit source]
Case definitions[edit | edit source]
Early descriptions of symptom exacerbation in ME focused on post-exertional muscle weakness. Renowned ME-expert Melvin Ramsay for example wrote:
"Muscle fatigability whereby, even after a minor degree of physical effort, three, four or five days or longer elapse before full muscle power is restored is unique and constitutes the sheet anchor of diagnosis. Without it I would be unwilling to diagnose a patient as suffering from ME."[64]
In a 1985 study Behan et al. noted that all of their patients “had the same primary symptom that of gross fatigue made worse by exercise".[134]
Formerly used to define Chronic fatigue syndrome[edit | edit source]
In the 1988 Holmes criteria for CFS, unexplained generalized muscle weakness was one of the 11 minor symptoms, yet it was fatigue that set the tone. Another minor symptom referred to "prolonged (24 hours or greater) generalized fatigue after levels of exercise that would have been easily tolerated in the patient's premorbid state".[135] PEM is not a mandatory symptom under the Holmes definition.
The wording "postexertional malaise" was used in the article Symptoms and signs of chronic fatigue syndrome by Anthony Komaroff and Dedra Buchwald, from 1991.[136]
The wording "postexertional malaise" was used as one of the 8 minor symptoms in the 1994 Fukuda criteria, but without further clarification of the term, except that it lasts more than 24 hours. PEM is not a mandatory symptom under the Fukuda criterion.
Retired criteria for Chronic fatigue syndrome[edit | edit source]
The Oxford criteria has been retired from use after a number of scientific critcisms were raised, including that it does not list PEM as a requirement or even a symptom.[137] Patients with the symptom of fatigue, which many illnesses and diseases have, are incorrectly given the CFS diagnosis in research studies when the Oxford criteria is used.
The United States National Institutes of Health (NIH) has issued a draft report that highlights the dire need for scientific research that will help find a cure for the millions of people suffering from myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) worldwide. The report also highlights the fact that the decades-old UK Royal Society of Medicine’s Oxford criteria for ME/CFS are severely “flawed,” and that continuing to use these criteria may “cause harm.” Further, the NIH report says that the Royal Society definition should “be retired” and replaced with a single case definition agreed to by the ME/CFS community.[138]
Currently used as the hallmark symptom defining ME/CFS[edit | edit source]
Post-exertional malaise was recognized as a symptom of chronic fatigue syndrome in the 1994 Fukuda criteria, but did not fully describe it, and only identified it as an optional symptom.[124]
The 2003 Canadian Consensus Criteria (CCC) for ME/CFS used PEM as the key compulsory symptom for diagnosis. The CCC's were the first criteria to stress that the onset of PEM could be delayed and to describe its debility as a flu-like distress.[48] PEM and/or post-exertional fatigue is a mandatory symptom under the CCC criterion.[139] The International Consensus Criteria for ME replaced post-exertional malaise with a similar symptom of post-exertional neuroimmune exhaustion, though this criteria is rarely used in clinical practice.[42]
The National Academy of Medicine 2015 report describes PEM more generally as "an exacerbation of some or all of an individual's ME/CFS symptoms that occurs after physical or cognitive exertion and leads to a reduction in functional ability." The report confirmed PEM as the hallmark symptom of ME/CFS and advised to rename the disease accordingly to Systemic Exertion Intolerance Disease (SEID).[41] PEM is a mandatory symptom under the SEID criterion, which was adopted by the CDC and is used as the current ME/CFS criteria.[3]
The UK uses the NHS diagnostic criteria, which were changed in 2021 to use post-exertional malaise as the hallmark symptom a required (compulsory) symptom that is needed for ME/CFS to be diagnosed.[3][9]
Currently used to define Myalgic encephalomyelitis[edit | edit source]
In 2011, the International Consensus Criteria (ICC) introduced the new term Post-Exertional Neuro-immune Exhaustion (PENE) to refer to the characteristic exercise and exertion intolerance of myalgic encephalomyelitis (ME) patients. It notes a delayed onset and prolonged recovery, and uses acute flu-like symptoms to describe PENE. By definition PENE results in a substantial reduction in functioning, as even simple activities of daily living can cause a relapse.[42] PENE is a mandatory symptom under the ICC criterion.
Long COVID[edit | edit source]
Post-exertional malaise is a potential symptom of Long COVID in the World Health Organization's definition.[140]
Psychological paradigm[edit | edit source]
Dismissed as disturbed effort perceptions or kinesiophobia[edit | edit source]

The existence of PEM as a distinctive and complex symptom of ME/CFS has been dismissed in early research into the disease. Some interpreted it as just fatigue after exercise[141], while others saw it as an artifact of disturbed effort perceptions[142][143][144] or an irrational fear of movement[145][146]. One example of this is the Tampa scale for kinesiophobia, adapted for chronic fatigue syndrome. Some of the questions in this scale ask about the experience of PEM such as: "If I were to try to overcome it, my symptoms would increase" or "my symptoms let me know when to stop exercising so that I do not harm myself". Yet these symptoms are classified as an indicator of irrational fear of movement and exercise, instead of PEM.[147]
Critique of the term[edit | edit source]
The name post-exertional malaise was introduced by the 1994 Fukuda criteria and had no prior medical meaning attached to it.[1] While in the scientific literature, the term has become the standard to describe the relapses ME/CFS patients suffer after exertion, patients argue that it trivializes their experience. The term malaise after all refers to "a general feeling of discomfort, illness, or unease whose exact cause is difficult to identify"[148]. Doctor of Public Health at Berkely, David Tuller, calls post-exertional malaise a "complete misnomer" arguing what ME/CFS patients experience "is much closer to a serious crash or relapse than a Victorian fainting spell."[149] ME/CFS patients usually use the abbreviation PEM or the term "crash" to describe their relapses.
Treatment[edit | edit source]
PEM has no known treatment. Some possible treatments have been hypothesized.
Notable studies[edit | edit source]
- 2021, Effects of Post-Exertional Malaise on Markers of Arterial Stiffness in Individuals with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome[150] - (Full text)
- 2020, The physiological time line of post-exertional malaise in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS)[6] - (Full text)
- 2020, Characterization of Post–exertional Malaise in Patients With Myalgic Encephalomyelitis/Chronic Fatigue Syndrome[5] (Full text)
- 2018, Comparing Post-Exertional Symptoms Following Serial Exercise Tests[151] - (Abstract)
- 2018, Deconstructing post-exertional malaise in myalgic encephalomyelitis/chronic fatigue syndrome: A patient-centered, cross-sectional survey[1] - (Full text)
- 2018, The development of an instrument to assess post-exertional malaise in patients with myalgic encephalomyelitis and chronic fatigue syndrome[8][7] - (Abstract) (Questionnaire)
- 2017, Symptom variability following acute exercise in myalgic encephalomyelitis/chronic fatigue syndrome: a perspective on measuring post-exertion malaise[152] - (Full text)
- 2016, Deconstructing post-exertional malaise: An exploratory factor analysis[47] - (Full Text)
- 2015, Myalgic Encephalomyelitis: Symptoms and Biomarkers[153] - (Full Text)
- 2015, Changes in Gut and Plasma Microbiome following Exercise Challenge in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS)[120] - (Full Text)
- 2015, Factor Analysis of the DePaul Symptom Questionnaire: Identifying Core Domains[154] (Full text) - assessed different types of post-exertional malaise
- 2013, Post-exertion malaise in chronic fatigue syndrome: symptoms and gene expression[30] - (Abstract)
- 2010, Postexertional Malaise in Women with Chronic Fatigue Syndrome[15] - (Abstract)
- 1999, Demonstration of delayed recovery from fatiguing exercise in chronic fatigue syndrome[155] - (Abstract)
- 1994, The chronic fatigue syndrome: a comprehensive approach to its definition and study[124] - (Full text)
Talks and interviews[edit | edit source]
- 2013, CFS gene expression after exercise (part 1)
- 2012, Top 10 Things You Should Know About Post-Exertional Relapse - University of the Pacific/Solve CFS - 2010 study, PEM in Women with CFS is discussed
See also[edit | edit source]
Learn more[edit | edit source]
- International CFS/ME Awareness Day - What Health (PEM Definition Included)
- Postexertion 'Crash,' not Fatigue per se, Marks Syndrome - Medscape
- Post-Exertional Malaise in Chronic Fatigue Syndrome
- Post-Exertional Malaise: Cause and Effect
- How to Best Recover From a Crash: the ME/CFS Community Reports - Health Rising
- The Exercise Intolerance in POTS, ME/CFS and Fibromyalgia Explained?
References[edit | edit source]
- ↑ Jump up to: 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 Chu, Lily; Valencia, Ian J.; Garvert, Donn W.; Montoya, Jose G. (2018). "Deconstructing post-exertional malaise in myalgic encephalomyelitis/chronic fatigue syndrome: A patient-centered, cross-sectional survey". PloS One. 13 (6): e0197811. doi:10.1371/journal.pone.0197811. ISSN 1932-6203. PMC 5983853. PMID 29856774.
- ↑ Jump up to: 2.0 2.1 2.2 Arroll, Megan A.; Attree, Elizabeth A.; O'Leary, John M.; Dancey, Christine P. (April 3, 2014). "The delayed fatigue effect in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS)". Fatigue: Biomedicine, Health & Behavior. 2 (2): 57–63. doi:10.1080/21641846.2014.892755. ISSN 2164-1846.
- ↑ Jump up to: 3.0 3.1 3.2 3.3 3.4 3.5 "Symptoms | Myalgic Encephalomyelitis/Chronic Fatigue Syndrome". Centers for Disease Control and Prevention. May 18, 2018. Retrieved November 21, 2018.
- ↑ Jump up to: 4.0 4.1 Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen (IQWiG) (17 April 2023). Myalgische Enzephalomyelitis / Chronic Fatigue Syndrome (ME/CFS): Aktueller Kenntnisstand [Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): current state of knowledge] (PDF) (in German). Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen. ISSN 1864-2500. Archived(PDF) from the original on 2 November 2023. Retrieved 8 November 2023.
- ↑ Jump up to: 5.0 5.1 5.2 5.3 5.4 Stussman, Barbara; Williams, Ashley; Snow, Joseph; Gavin, Angelique; Scott, Remle; Nath, Avindra; Walitt, Brian (2020). "Characterization of Post–exertional Malaise in Patients With Myalgic Encephalomyelitis/Chronic Fatigue Syndrome". Frontiers in Neurology. 11. doi:10.3389/fneur.2020.01025. ISSN 1664-2295.
- ↑ Jump up to: 6.0 6.1 Hodges, Lynette; Nielsen, Tessa; Cochrane, Darryl; Baken, Donald (May 2020). "The physiological time line of post‐exertional malaise in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS)". Translational Sports Medicine. 3 (3): 243–249. doi:10.1002/tsm2.133. ISSN 2573-8488.
- ↑ Jump up to: 7.0 7.1 Jason, Leonard A; Holtzman, Carly S; Sunnquist, Madison; Cotler, Joseph (2018). "The development of an instrument to assess post-exertional malaise in patients with myalgic encephalomyelitis and chronic fatigue syndrome" (PDF). Journal of Health Psychology: 4–5. doi:10.1177/1359105318805819/suppl_file/appendix.__the_development_of_a_comprehensive_measure_of_post-exertional_malaise.8.20.2018.pdf.
- ↑ Jump up to: 8.0 8.1 8.2 8.3 8.4 Jason, Leonard A; Holtzman, Carly S; Sunnquist, Madison; Cotler, Joseph (October 24, 2018). "The development of an instrument to assess post-exertional malaise in patients with myalgic encephalomyelitis and chronic fatigue syndrome". Journal of Health Psychology: 1359105318805819. doi:10.1177/1359105318805819. ISSN 1359-1053.
- ↑ Jump up to: 9.0 9.1 9.2 Strain, David (April 21, 2022). "Key learning points: revised NICE guidance on ME/CFS". Guidelines in Practice. Retrieved June 13, 2022.
- ↑ Robb-Nicholson, L. C.; Daltroy, L.; Eaton, H.; Gall, V.; Wright, E.; Hartley, L.H.; Schur, P.H.; Liang, M.H. (December 1989). "Effects of aerobic conditioning in lupus fatigue: a pilot study". British Journal of Rheumatology. 28 (6): 500–505. ISSN 0263-7103. PMID 2590802.
- ↑ Mostert, S.; Kesselring, J. (April 2002). "Effects of a short-term exercise training program on aerobic fitness, fatigue, health perception and activity level of subjects with multiple sclerosis". Multiple Sclerosis. 8 (2): 161–168. doi:10.1191/1352458502ms779oa. ISSN 1352-4585. PMID 11990874.
- ↑ Jump up to: 12.0 12.1 12.2 12.3 NINDS CDE Project Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) Post Exertional Malaise Subgroup. "Post-exertional Malaise Subgroup Statement of Purview" (PDF). National Institutes of Health. Retrieved February 21, 2021.
- ↑ "What you need to know about exercise and chronic disease". Mayo Clinic. Retrieved October 10, 2018.
- ↑ Nijs, Jo; Almond, Freya; De Becker, Pascale; Truijen, Steven; Paul, Lorna (May 2008). "Can exercise limits prevent post-exertional malaise in chronic fatigue syndrome? An uncontrolled clinical trial". Clinical Rehabilitation. 22 (5): 426–435. doi:10.1177/0269215507084410. ISSN 0269-2155. PMID 18441039.
- ↑ Jump up to: 15.0 15.1 15.2 15.3 15.4 15.5 15.6 15.7 VanNess, J. Mark; Stevens, Staci R.; Bateman, Lucinda; Stiles, Travis L.; Snell, Christopher R. (February 2010). "Postexertional malaise in women with chronic fatigue syndrome". Journal of Women's Health. 19 (2): 239–244. doi:10.1089/jwh.2009.1507. ISSN 1931-843X. PMID 20095909.
- ↑ The Voice of the Patient. | Chronic Fatigue Syndrome and Myalgic Encephalomyelitis (PDF), Center for Drug Evaluation and Research (CDER) | U.S. Food and Drug Administration., September 2013
- ↑ "Recommendations | Myalgic encephalomyelitis (or encephalopathy)/chronic fatigue syndrome: diagnosis and management | Guidance | NICE". www.nice.org.uk. October 29, 2021. Retrieved May 20, 2024.
- ↑ Habermann-Horstmeier L, Horstmeier LM. Auswirkungen der Qualität der Arzt-Patient-Beziehung auf die Gesundheit von erwachsenen ME/CFS-Erkrankten : Eine qualitative Public-Health-Studie aus Patientensicht [Implications of the quality of the doctor-patient relationship on health in adult ME/CFS patients. A qualitative public health study from a patien perspective]. MMW Fortschr Med. 2023 Dec;165(Suppl 5):16-27. German. doi: 10.1007/s15006-023-2894-z. PMID: 38062324.
- ↑ Thoma, Manuel; Froehlich, Laura; Hattesohl, Daniel B. R.; Quante, Sonja; Jason, Leonard A.; Scheibenbogen, Carmen (January 2024). "Why the Psychosomatic View on Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Is Inconsistent with Current Evidence and Harmful to Patients". Medicina. 60 (1): 83. doi:10.3390/medicina60010083. ISSN 1648-9144. PMC 10819994. PMID 38256344.
- ↑ NICE Guideline Development Group (October 29, 2021). "Myalgic Encephalomyelitis (or Encephalopathy)/Chronic Fatigue Syndrome:diagnosis and management. NICE guideline". National Institute for Health and Care Excellence.
- ↑ Jump up to: 21.0 21.1 21.2 Kaufman, David (October 16, 2018). "Diagnosis and Management of Myalgic Encephalomyelitis and Chronic Fatigue Syndrome". YouTube. Unrest Film.
- ↑ Jump up to: 22.0 22.1 22.2 22.3 22.4 22.5 22.6 22.7 Cotler, Joseph; Holtzman, Carly; Dudun, Catherine; Jason, Leonard A. (September 11, 2018). "A Brief Questionnaire to Assess Post-Exertional Malaise". Diagnostics (Basel, Switzerland). 8 (3). doi:10.3390/diagnostics8030066. ISSN 2075-4418. PMID 30208578.
- ↑ Vink, Mark (September 10, 2015). "The Aerobic Energy Production and the Lactic Acid Excretion are both Impeded in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome". Journal of Neurology and Neurobiology ( ISSN 2379-7150 ). 1. doi:10.16966/2379-7150.112.
- ↑ Carruthers, BM; van de Sande, MI; De Meirleir, KL; Klimas, NG; Broderick, G; Mitchell, T; Staines, D; Powles, ACP; Speight, N; Vallings, R; Bateman, L; Bell, DS; Carlo-Stella, N; Chia, J; Darragh, A; Gerken, A; Jo, D; Lewis, DP; Light, AR; Light, KC; Marshall-Gradisnik, S; McLaren-Howard, J; Mena, I; Miwa, K; Murovska, M; Stevens, SR (2012), Myalgic encephalomyelitis: Adult & Paediatric: International Consensus Primer for Medical Practitioners (PDF), ISBN 978-0-9739335-3-6
- ↑ Jump up to: 25.0 25.1 Blackwood, S.; MacHale, S.; Power, M.; Goodwin, G.; Lawrie, S. (October 1998). "Effects of exercise on cognitive and motor function in chronic fatigue syndrome and depression". Journal of Neurology, Neurosurgery, and Psychiatry. 65 (4): 541–546. ISSN 0022-3050. PMC 2170292. PMID 9771781.
- ↑ Jump up to: 26.0 26.1 Cook, Dane B; Vernon, Suzanne D. (May 1, 2017). "Neural consequences of post-exertion malaise in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome". Brain, Behavior, and Immunity. 62: 87–99. doi:10.1016/j.bbi.2017.02.009. ISSN 0889-1591.
- ↑ Jump up to: 27.0 27.1 Nijs, Jo; Nees, Andrea; Paul, Lorna; De Kooning, Margot; Ickmans, Kelly; Meeus, Mira; Van Oosterwijck, Jessica (2014). "Altered immune response to exercise in patients with chronic fatigue syndrome/myalgic encephalomyelitis: a systematic literature review". Exercise Immunology Review. 20: 94–116. ISSN 1077-5552. PMID 24974723.
- ↑ Jump up to: 28.0 28.1 Light, Alan R.; White, Andrea T.; Hughen, Ronald W.; Light, Kathleen C. (October 2009). "Moderate Exercise Increases Expression for Sensory, Adrenergic, and Immune Genes in Chronic Fatigue Syndrome Patients But Not in Normal Subjects". The Journal of Pain. 10 (10): 1099–1112. doi:10.1016/j.jpain.2009.06.003. ISSN 1526-5900.
- ↑ Jump up to: 29.0 29.1 Light, A.R.; Bateman, L.; Jo, D.; Hughen, R.W.; VanHaitsma, T.A.; White, A.T.; Light, K.C. (July 13, 2011). "Gene expression alterations at baseline and following moderate exercise in patients with Chronic Fatigue Syndrome and Fibromyalgia Syndrome". Journal of Internal Medicine. 271 (1): 64–81. doi:10.1111/j.1365-2796.2011.02405.x. ISSN 0954-6820.
- ↑ Jump up to: 30.0 30.1 30.2 Meyer, Jacob D.; Light, Alan R.; Shukla, Sanjay K.; Clevidence, Derek; Yale, Steven; Stegner, Aaron J.; Cook, Dane B. (October 2013). "Post-exertion malaise in chronic fatigue syndrome: symptoms and gene expression". Fatigue: Biomedicine, Health & Behavior. 1 (4): 190–209. doi:10.1080/21641846.2013.838444. ISSN 2164-1846.
- ↑ Jump up to: 31.0 31.1 White, A. T.; Light, A.R.; Hughen, R.W.; VanHaitsma, T.A.; Light, K.C. (December 30, 2011). "Differences in Metabolite-Detecting, Adrenergic, and Immune Gene Expression After Moderate Exercise in Patients With Chronic Fatigue Syndrome, Patients With Multiple Sclerosis, and Healthy Controls". Psychosomatic Medicine. 74 (1): 46–54. doi:10.1097/psy.0b013e31824152ed. ISSN 0033-3174.
- ↑ Jump up to: 32.0 32.1 Whiteside, Alan; Hansen, Stig; Chaudhuri, Abhijit (June 2004). "Exercise lowers pain threshold in chronic fatigue syndrome". Pain. 109 (3): 497–499. doi:10.1016/j.pain.2004.02.029. ISSN 0304-3959.
- ↑ Jump up to: 33.0 33.1 Meeus, M; Roussel, NA; Truijen, S (2010). "Reduced pressure pain thresholds in response to exercise in chronic fatigue syndrome but not in chronic low back pain: An experimental study". Journal of Rehabilitation Medicine. 42 (9): 884–890. doi:10.2340/16501977-0595. ISSN 1650-1977.
- ↑ Jump up to: 34.0 34.1 Van Oosterwijck, J.; Nijs, J.; Meeus, M.; Lefever, I.; Huybrechts, L.; Lambrecht, L.; Paul, L. (March 3, 2010). "Pain inhibition and postexertional malaise in myalgic encephalomyelitis/chronic fatigue syndrome: An experimental study". Journal of Internal Medicine. 268 (3): 265–278. doi:10.1111/j.1365-2796.2010.02228.x. ISSN 0954-6820.
- ↑ Jump up to: 35.0 35.1 Stevens, Staci; Snell, Chris; Stevens, Jared; Keller, Betsy; VanNess, J. Mark (2018). "Cardiopulmonary Exercise Test Methodology for Assessing Exertion Intolerance in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome". Frontiers in Pediatrics. 6. doi:10.3389/fped.2018.00242. ISSN 2296-2360.
- ↑ Jump up to: 36.0 36.1 36.2 Snell, Christopher R.; Stevens, Staci R; Davenport, Todd E.; VanNess, J. Mark (June 27, 2013). "Discriminative Validity of Metabolic and Workload Measurements for Identifying People With Chronic Fatigue Syndrome". Physical Therapy. 93 (11): 1484–1492. doi:10.2522/ptj.20110368. ISSN 0031-9023.
- ↑ Jump up to: 37.0 37.1 Vermeulen, Ruud CW; Kurk, Ruud M; Visser, Frans C; Sluiter, Wim; Scholte, Hans R (2010). "Patients with chronic fatigue syndrome performed worse than controls in a controlled repeated exercise study despite a normal oxidative phosphorylation capacity". Journal of Translational Medicine. 8 (1): 93. doi:10.1186/1479-5876-8-93. ISSN 1479-5876.
- ↑ Jump up to: 38.0 38.1 38.2 Keller, Betsy A.; Pryor, John Luke; Giloteaux, Ludovic (April 23, 2014). "Inability of myalgic encephalomyelitis/chronic fatigue syndrome patients to reproduce VO₂peak indicates functional impairment". Journal of Translational Medicine. 12: 104. doi:10.1186/1479-5876-12-104. ISSN 1479-5876. PMC 4004422. PMID 24755065.
- ↑ Jump up to: 39.0 39.1 VanNess, J Mark; Snell, Christopher R; Stevens, Staci R (2007). "Diminished Cardiopulmonary Capacity During Post-Exertional Malaise". Journal of Chronic Fatigue Syndrome. 14 (2): 77–85. doi:10.1300/J092v14n02_07.
- ↑ Jump up to: 40.0 40.1 40.2 Hodges, L. D.; Nielsen, T.; Baken, D. (July 2018). "Physiological measures in participants with chronic fatigue syndrome, multiple sclerosis and healthy controls following repeated exercise: a pilot study". Clinical Physiology and Functional Imaging. 38 (4): 639–644. doi:10.1111/cpf.12460. ISSN 1475-097X. PMID 28782878.
- ↑ Jump up to: 41.0 41.1 41.2 41.3 41.4 41.5 41.6 41.7 Committee on the Diagnostic Criteria for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome; Board on the Health of Select Populations; Institute of Medicine (2015). Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining an Illness. The National Academies Collection: Reports funded by National Institutes of Health. Washington (DC): National Academies Press (US). ISBN 9780309316897. PMID 25695122.
- ↑ Jump up to: 42.0 42.1 42.2 42.3 42.4 Carruthers, Bruce M.; van de Sande, Marjorie I.; De Meirleir, Kenny L.; Klimas, Nancy G.; Broderick, Gordon; Mitchell, Terry; Staines, Donald; Powles, A.C. Peter; Speight, Nigel; Vallings, Rosamund; Bateman, Lucinda; Baumgarten-Austrheim, Barbara; Bell, David; Carlo-Stella, Nicoletta; Chia, John; Darragh, Austin; Jo, Daehyun; Lewis, Donald; Light, Alan; Marshall-Gradisnik, Sonya; Mena, Ismael; Mikovits, Judy; Miwa, Kunihisa; Murovska, Modra; Pall, Martin; Stevens, Staci (August 22, 2011). "Myalgic encephalomyelitis: International Consensus Criteria". Journal of Internal Medicine. 270 (4): 327–338. doi:10.1111/j.1365-2796.2011.02428.x. ISSN 0954-6820. PMC 3427890. PMID 21777306.
- ↑ Loy, Bryan D.; O'Connor, Patrick J.; Dishman, Rodney K. (October 2013). "The effect of a single bout of exercise on energy and fatigue states: a systematic review and meta-analysis". Fatigue: Biomedicine, Health & Behavior. 1 (4): 223–242. doi:10.1080/21641846.2013.843266. ISSN 2164-1846.
- ↑ US District Court District of New Jersey, Brian Vastag v. Prudential Insurance Company of America, Civ. No. 15-6197 (KSH) (CLW) (D.N.J. May. 31, 2018) (PDF)
- ↑ Tillman, Adriane (June 4, 2018). "Victory for ME Disability Claim - U.S. Court Upholds Plaintiff's Lawsuit After Being Denied Disability". #MEAction. Retrieved February 2, 2019.
- ↑ Jump up to: 46.0 46.1 Jason, Leonard A.; Evans, Meredyth; So, Suzanna; Scott, Jilian; Brown, Abigail (2015). "Problems in Defining Post-Exertional Malaise". Journal of prevention & intervention in the community. 43 (1): 20–31. doi:10.1080/10852352.2014.973239. ISSN 1085-2352. PMC 4295644. PMID 25584525.
- ↑ Jump up to: 47.0 47.1 47.2 47.3 McManimen, SL; Sunnquist, ML; Jason, LA (2016). "Deconstructing post-exertional malaise: An exploratory factor analysis". Journal of Health Psychology. 24 (2): 188–198. doi:10.1177/1359105316664139. PMC 5325824. PMID 27557649.
- ↑ Jump up to: 48.0 48.1 48.2 Carruthers, Bruce M.; Jain, Anil Kumar; De Meirleir, Kenny L.; Peterson, Daniel L.; Klimas, Nancy G.; Lerner, A. Martin; Bested, Alison C.; Flor-Henry, Pierre; Joshi, Pradip (January 2003). "Myalgic Encephalomyelitis/Chronic Fatigue Syndrome". Journal of Chronic Fatigue Syndrome. 11 (1): 7–115. doi:10.1300/j092v11n01_02. ISSN 1057-3321.
- ↑ Jump up to: 49.0 49.1 49.2 Yoshiuchi, Kazuhiro; Cook, Dane B.; Ohashi, Kyoko; Kumano, Hiroaki; Kuboki, Tomifusa; Yamamoto, Yoshiharu; Natelson, Benjamin H. (December 5, 2007). "A real-time assessment of the effect of exercise in chronic fatigue syndrome". Physiology & Behavior. 92 (5): 963–968. doi:10.1016/j.physbeh.2007.07.001. ISSN 0031-9384. PMC 2170105. PMID 17655887.
- ↑ Croxall, Jack (January 17, 2019). "I think #TwoFacesofME is a really important hashtag. We're only out and about at our best, and our (more frequent) worst often remains hidden. I'm convinced it's why #MEcfs research funding is so low - the problem isn't visible enough. I'm seriously ill in both these photos.pic.twitter.com/hNjK5140kv". twitter.com. Retrieved January 17, 2019.
- ↑ Kaise 🥄, Mx (January 17, 2019). "#twofacesofME #MEcfs - It's not always easy to predict when you'll take a turn for the worst. It's not even easy to tell how you'll feel when you crash. You just know that at some point in the future, it's going to happen.pic.twitter.com/nTJVG63jRm". twitter. Retrieved January 17, 2019.
- ↑ Francis, Rachel (January 17, 2019). "#TwoFacesofME Workday me vs weekend me. I'm so grateful that most of the time I can work, but losing out on family time on a weekend to recover is hard.pic.twitter.com/qZNiQaVHyD". twitter. Retrieved January 17, 2019.
- ↑ Karen (January 17, 2019). "First photo, me in my wheelchair on a rare trip out. Second photo, the inevitable crash. Eye half closed, slurred speech, dizzy, weak etc., etc. #TwoFacesofMEpic.twitter.com/P2OPnnpQvF". Twitter. Retrieved January 17, 2019.
- ↑ Köhler, Henry (January 17, 2019). "#TwoFacesofME First photo from the morning, the other one from the afternoon ( when I failed to nap 30-60 minutes). I am Not severely ill, and my life is ok, even do I wish that one day science will help me & all the #MeCfs sufferers around the globe.pic.twitter.com/wmhfHcfP0p". Twitter. Retrieved January 17, 2019.
- ↑ CFDA Awareness# (January 15, 2019). "What the don't two faces of M.E.pic.twitter.com/DYZVhtyrG5". Twitter. Retrieved January 17, 2019.
- ↑ Ceiba 🌳Koru 🌀 (January 17, 2019). "Joining #pwME sharing #TwoFacesofME for #MEAwareness, 1: a fall day when I got outside (but ended up in bed a week), 2: what no one sees (constant pain, post exertional malaise & sleep deprivation). Bonus for comparison: snowboarding shortly after cancer treatment (but before ME)pic.twitter.com/EpsMLT8E11". Twitter. Retrieved January 17, 2019.
- ↑ "Fatigo_MECFS on Twitter". Twitter. Retrieved October 11, 2018.
- ↑ Spotila, Jennifer. "Post-Exertional Malaise II: Perception and Reality By Jennifer M. Spotila, J.D." Phoenix Rising. Retrieved October 10, 2018.
- ↑ Larun, Lillebeth; Malterud, Kirsti (May 2011). "Finding the right balance of physical activity: a focus group study about experiences among patients with chronic fatigue syndrome". Patient Education and Counseling. 83 (2): 222–226. doi:10.1016/j.pec.2010.05.027. ISSN 1873-5134. PMID 20580520.
- ↑ Goudsmit, Ellen M.; Nijs, Jo; Jason, Leonard A.; Wallman, Karen E. (2012). "Pacing as a strategy to improve energy management in myalgic encephalomyelitis/chronic fatigue syndrome: a consensus document". Disability and Rehabilitation. 34 (13): 1140–1147. doi:10.3109/09638288.2011.635746. ISSN 1464-5165. PMID 22181560.
- ↑ Jump up to: 61.0 61.1 61.2 "S4ME: Submission to the public review on Common Data Elements for ME/CFS: Concerns with the proposed measure of post-exertional malaise". Science for ME. Retrieved October 10, 2018.
- ↑ Bazelmans, Ellen; Bleijenberg, Gijs; Voeten, Marinus J.M.; van der Meer, Jos W.M.; Folgering, Hans (October 2005). "Impact of a maximal exercise test on symptoms and activity in chronic fatigue syndrome". Journal of Psychosomatic Research. 59 (4): 201–208. doi:10.1016/j.jpsychores.2005.04.003. ISSN 0022-3999. PMID 16223622.
- ↑ Lapp, C.W. (July 1997). "Exercise limits in chronic fatigue syndrome". The American Journal of Medicine. 103 (1): 83–84. ISSN 0002-9343. PMID 9236491.
- ↑ Jump up to: 64.0 64.1 64.2 Ramsay M. (1988). Myalgic Encephalomyelitis and Postviral Fatigue States: The Saga of Royal Free Disease. Gower Medical Publishing. Second edition.
- ↑ "Dance hermit '16 vs. Sumo Baby (part 1) | Anil van der Zee". anilvanderzee.com. Retrieved October 13, 2018.
- ↑ Jump up to: 66.0 66.1 66.2 NINDS/CDC Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) Post-Exertional Malaise Subgroup Draft Recommendations Public Review Comments Due January 31, 2018
- ↑ "Unraveling Post-exertional Malaise By Jennifer M. Spotila". Phoenix Rising. Retrieved October 10, 2018.
- ↑ Bell, David S (June 25, 2016). "ME/CFS in Children". Open Medicine Foundation. Retrieved August 11, 2018.
- ↑ Wagner, Dieter; Nisenbaum, Rosane; Heim, Christine; Jones, James F.; Unger, Elizabeth R.; Reeves, William C. (July 22, 2005). "Psychometric properties of the CDC Symptom Inventory for assessment of chronic fatigue syndrome". Population Health Metrics. 3: 8. doi:10.1186/1478-7954-3-8. ISSN 1478-7954. PMC 1183246. PMID 16042777.
- ↑ Vollmer-Conna, Uté; Aslakson, Eric; White, Peter D (April 2006). "An empirical delineation of the heterogeneity of chronic unexplained fatigue in women". Pharmacogenomics. 7 (3): 355–364. doi:10.2217/14622416.7.3.355. ISSN 1462-2416.
- ↑ Jason, Leonard A. (February 2011). "Natural History of Chronic Fatigue Syndrome". Rehabilitation psychology. 56 (1): 32–42. doi:10.1037/a0022595. ISSN 0090-5550. PMC 3171164. PMID 21401284.
- ↑ Jason, Leonard A.; Kot, Bobby; Sunnquist, Madison; Brown, Abigail; Reed, Jordan; Furst, Jacob; Newton, Julia L.; Strand, Elin Bolle; Vernon, Suzanne D. (April 1, 2014). "Comparing and Contrasting Consensus versus Empirical Domains". Fatigue: Biomedicine, Health & Behavior. 3 (2): 63–74. doi:10.1080/21641846.2015.1017344. ISSN 2164-1846. PMC 4788637. PMID 26977374.
- ↑ Jason, Leonard A.; Sunnquist, Madison; Brown, Abigail; Evans, Meredyth; Vernon, Suzanne D.; Furst, Jacob; Simonis, Valerie (January 1, 2014). "Examining case definition criteria for chronic fatigue syndrome and myalgic encephalomyelitis". Fatigue: Biomedicine, Health & Behavior. 2 (1): 40–56. doi:10.1080/21641846.2013.862993. ISSN 2164-1846. PMC 3912876. PMID 24511456.
- ↑ Jason, Leonard A.; Kot, Bobby; Sunnquist, Madison; Brown, Abigail; Evans, Meredyth; Jantke, Rachel; Williams, Yolonda; Furst, Jacob; Vernon, Suzanne D. (2015). "Chronic Fatigue Syndrome and Myalgic Encephalomyelitis: Toward An Empirical Case Definition". Health Psychology and Behavioral Medicine. 3 (1): 82–93. doi:10.1080/21642850.2015.1014489. ISSN 2164-2850. PMC 4443921. PMID 26029488.
- ↑ Maes, Michael; Twisk, Frank N.M.; Johnson, Cort (December 30, 2012). "Myalgic Encephalomyelitis (ME), Chronic Fatigue Syndrome (CFS), and Chronic Fatigue (CF) are distinguished accurately: results of supervised learning techniques applied on clinical and inflammatory data". Psychiatry Research. 200 (2–3): 754–760. doi:10.1016/j.psychres.2012.03.031. ISSN 1872-7123. PMID 22521895.
- ↑ Jump up to: 76.0 76.1 Komaroff, A. L.; Fagioli, L.R.; Geiger, A.M.; Doolittle, T.H.; Lee, J.; Kornish, R.J.; Gleit, M.A.; Guerriero, R.T. (January 1996). "An examination of the working case definition of chronic fatigue syndrome". The American Journal of Medicine. 100 (1): 56–64. ISSN 0002-9343. PMID 8579088.
- ↑ Jason, L.A.; Ohanian, D.; Brown, A.; Sunnquist, M.; McManimen, S.; Klebek, L.; Fox, P.; Sorenson, M. (2017). "Differentiating Multiple Sclerosis from Myalgic Encephalomyelitis and Chronic Fatigue Syndrome". Insights in Biomedicine. 2 (2). doi:10.21767/2572-5610.10027. ISSN 2572-5610. PMC 5800741. PMID 29430570.
- ↑ Hawk, Caroline; Jason, Leonard A.; Torres-Harding, Susan (2006). "Differential diagnosis of chronic fatigue syndrome and major depressive disorder". International Journal of Behavioral Medicine. 13 (3): 244–251. doi:10.1207/s15327558ijbm1303_8. ISSN 1070-5503. PMID 17078775.
- ↑ White, PD (1995). "The validity and reliability of the fatigue syndrome that follows glandular fever". Journal of Psychological Medicine. 25 (5): 917–24. doi:10.1017/s0033291700037405. Retrieved October 24, 2018.
- ↑ Baraniuk, James N.; Shivapurkar, Narayan (November 10, 2017). "Exercise – induced changes in cerebrospinal fluid miRNAs in Gulf War Illness, Chronic Fatigue Syndrome and sedentary control subjects". Scientific Reports. 7 (1). doi:10.1038/s41598-017-15383-9. ISSN 2045-2322.
- ↑ Smylie, Anne Liese; Broderick, Gordon; Fernandes, Henrique; Razdan, Shirin; Barnes, Zachary; Collado, Fanny; Sol, Connie; Fletcher, Mary Ann; Klimas, Nancy (June 25, 2013). "A comparison of sex-specific immune signatures in Gulf War illness and chronic fatigue syndrome". BMC immunology. 14: 29. doi:10.1186/1471-2172-14-29. ISSN 1471-2172. PMC 3698072. PMID 23800166.
- ↑ Jump up to: 82.0 82.1 82.2 Washington, Stuart D.; Rayhan, Rakib U.; Garner, Richard; Provenzano, Destie; Zajur, Kristina; Addiego, Florencia Martinez; VanMeter, John W.; Baraniuk, James N. (July 1, 2020). "Exercise alters brain activation in Gulf War Illness and Myalgic Encephalomyelitis/Chronic Fatigue Syndrome". Brain Communications. 2 (2). doi:10.1093/braincomms/fcaa070.
- ↑ Keech, Andrew; Vollmer-Conna, Ute; Barry, Benjamin K.; Lloyd, Andrew R. (2016). "Gene Expression in Response to Exercise in Patients with Chronic Fatigue Syndrome: A Pilot Study". Frontiers in Physiology. 7: 421. doi:10.3389/fphys.2016.00421. ISSN 1664-042X. PMC 5031769. PMID 27713703.
- ↑ Jammes, Y.; Steinberg, J.G.; Mambrini, O.; Brégeon, F.; Delliaux, S. (March 2005). "Chronic fatigue syndrome: assessment of increased oxidative stress and altered muscle excitability in response to incremental exercise". Journal of Internal Medicine. 257 (3): 299–310. doi:10.1111/j.1365-2796.2005.01452.x. ISSN 0954-6820. PMID 15715687.
- ↑ Jump up to: 85.0 85.1 Jammes, Y.; Steinberg, J.G.; Delliaux, S.; Brégeon, F. (August 2009). "Chronic fatigue syndrome combines increased exercise-induced oxidative stress and reduced cytokine and Hsp responses". Journal of Internal Medicine. 266 (2): 196–206. doi:10.1111/j.1365-2796.2009.02079.x. ISSN 1365-2796. PMID 19457057.
- ↑ Jammes, Y.; Steinberg, J.G.; Delliaux, S. (July 2012). "Chronic fatigue syndrome: acute infection and history of physical activity affect resting levels and response to exercise of plasma oxidant/antioxidant status and heat shock proteins". Journal of Internal Medicine. 272 (1): 74–84. doi:10.1111/j.1365-2796.2011.02488.x. ISSN 1365-2796. PMID 22112145.
- ↑ Thambirajah, Anita A.; Sleigh, Kenna; Stiver, H. Grant; Chow, Anthony W. (December 1, 2008). "Differential heat shock protein responses to strenuous standardized exercise in chronic fatigue syndrome patients and matched healthy controls". Clinical and Investigative Medicine. Medecine Clinique Et Experimentale. 31 (6): E319–327. ISSN 1488-2353. PMID 19032901.
- ↑ Sorensen, Bristol; Streib, Joanne E.; Strand, Matthew; Make, Barry; Giclas, Patricia C.; Fleshner, Monika; Jones, James F. (August 2003). "Complement activation in a model of chronic fatigue syndrome". The Journal of Allergy and Clinical Immunology. 112 (2): 397–403. ISSN 0091-6749. PMID 12897748.
- ↑ Sorensen, Bristol; Jones, James F; Vernon, Suzanne D; Rajeevan, Mangalathu S (January 2009). "Transcriptional Control of Complement Activation in an Exercise Model of Chronic Fatigue Syndrome". Molecular Medicine. 15 (1–2): 34–42. doi:10.2119/molmed.2008.00098. PMC 2583111. PMID 19015737.
- ↑ Nijs, J.; Van Oosterwijck, J.; Meeus, M.; Lambrecht, L.; Metzger, K.; Frémont, M.; Paul, L. (April 2010). "Unravelling the nature of postexertional malaise in myalgic encephalomyelitis/chronic fatigue syndrome: the role of elastase, complement C4a and interleukin-1β". Journal of Internal Medicine. 267 (4): 418–435. doi:10.1111/j.1365-2796.2009.02178.x. ISSN 0954-6820.
- ↑ Peterson, P. K.; Sirr, S.A.; Grammith, F.C.; Schenck, C.H.; Pheley, A.M.; Hu, S.; Chao, C.C. (March 1994). "Effects of mild exercise on cytokines and cerebral blood flow in chronic fatigue syndrome patients". Clinical and Diagnostic Laboratory Immunology. 1 (2): 222–226. ISSN 1071-412X. PMID 7496949.
- ↑ Lloyd, A.; Gandevia, S.; Brockman, A.; Hales, J.; Wakefield, D. (January 1994). "Cytokine production and fatigue in patients with chronic fatigue syndrome and healthy control subjects in response to exercise". Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America. 18 (Suppl 1): S142–146. ISSN 1058-4838. PMID 8148442.
- ↑ LaManca, J.J.; Sisto, S.A.; Zhou, X. D.; Ottenweller, J.E.; Cook, S.; Peckerman, A.; Zhang, Q.; Denny, T.N.; Gause, W.C. (March 1999). "Immunological response in chronic fatigue syndrome following a graded exercise test to exhaustion". Journal of Clinical Immunology. 19 (2): 135–142. ISSN 0271-9142. PMID 10226888.
- ↑ Cannon, J. G.; Angel, J.B.; Abad, L.W.; Vannier, E.; Mileno, M. D.; Fagioli, L.; Wolff, S.M.; Komaroff, A.L. (May 1997). "Interleukin-1 beta, interleukin-1 receptor antagonist, and soluble interleukin-1 receptor type II secretion in chronic fatigue syndrome". Journal of Clinical Immunology. 17 (3): 253–261. ISSN 0271-9142. PMID 9168406.
- ↑ Gupta, S.; Aggarwal, S.; Starr, A. (February 1999). "Increased production of interleukin-6 by adherent and non-adherent mononuclear cells during 'natural fatigue' but not following 'experimental fatigue' in patients with chronic fatigue syndrome". International Journal of Molecular Medicine. 3 (2): 209–213. ISSN 1107-3756. PMID 9917531.
- ↑ Cannon, J. G.; Angel, J.B.; Ball, R.W.; Abad, L.W.; Fagioli, L.; Komaroff, A.L. (November 1999). "Acute phase responses and cytokine secretion in chronic fatigue syndrome". Journal of Clinical Immunology. 19 (6): 414–421. ISSN 0271-9142. PMID 10634215.
- ↑ Robinson, M.; Gray, S.R.; Watson, M.S.; Kennedy, G.; Hill, A.; Belch, J.J.F.; Nimmo, M.A. (April 2010). "Plasma IL-6, its soluble receptors and F2-isoprostanes at rest and during exercise in chronic fatigue syndrome". Scandinavian Journal of Medicine & Science in Sports. 20 (2): 282–290. doi:10.1111/j.1600-0838.2009.00895.x. ISSN 1600-0838. PMID 19422646.
- ↑ Jump up to: 98.0 98.1 White, Andrea T.; Light, Alan R.; Hughen, Ronald W.; Bateman, Lucinda; Martins, Thomas B.; Hill, Harry R.; Light, Kathleen C. (July 1, 2010). "Severity of symptom flare after moderate exercise is linked to cytokine activity in chronic fatigue syndrome". Psychophysiology. 47 (4): 615–624. doi:10.1111/j.1469-8986.2010.00978.x. ISSN 1540-5958. PMC 4378647. PMID 20230500.
- ↑ Jump up to: 99.0 99.1 Moneghetti, Kegan J.; Skhiri, Mehdi; Contrepois, Kévin; Kobayashi, Yukari; Maecker, Holden; Davis, Mark; Snyder, Michael; Haddad, Francois; Montoya, Jose G. (February 9, 2018). "Value of Circulating Cytokine Profiling During Submaximal Exercise Testing in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome". Scientific Reports. 8 (1). doi:10.1038/s41598-018-20941-w. ISSN 2045-2322.
- ↑ Montague, T.J.; Marrie, T.J.; Klassen, G.A.; Bewick, D.J.; Horacek, B.M. (April 1989). "Cardiac function at rest and with exercise in the chronic fatigue syndrome". Chest. 95 (4): 779–784. ISSN 0012-3692. PMID 2924607.
- ↑ Gibson, H; Carroll, N; Clague, J E; Edwards, R H (September 1993). "Exercise performance and fatiguability in patients with chronic fatigue syndrome". Journal of Neurology, Neurosurgery, and Psychiatry. 56 (9): 993–998. ISSN 0022-3050. PMID 8410041.
- ↑ Sisto, Sue Ann; LaManca, John; Cordero, Douglas L.; Bergen, Michael T.; Ellis, Steven P.; Drastal, Susan; Boda, Wanda L.; Tapp, Walter N.; Natelson, Benjamin H. (June 1996). "Metabolic and cardiovascular effects of a progressive exercise test in patients with chronic fatigue syndrome". The American Journal of Medicine. 100 (6): 634–640. doi:10.1016/S0002-9343(96)00041-1. ISSN 0002-9343.
- ↑ Rowbottom, David; Keast, David; Pervan, Zhukov; Morton, Alan (January 1998). "The Physiological Response to Exercise in Chronic Fatigue Syndrome". Journal of Chronic Fatigue Syndrome. 4 (2): 33–49. doi:10.1300/j092v04n02_04. ISSN 1057-3321.
- ↑ De Becker, P.; Roeykens, J.; Reynders, M.; McGregor, N.; De Meirleir, K. (November 27, 2000). "Exercise capacity in chronic fatigue syndrome". Archives of Internal Medicine. 160 (21): 3270–3277. ISSN 0003-9926. PMID 11088089.
- ↑ Van Oosterwijck, J.; Marusic, U.; De Wandele, I.; Meeus, M.; Paul, L.; Lambrecht, L.; Moorkens, G.; Nijs, J. (May 2015). "Reduced parasympathetic reactivation during recovery from exercise in myalgic encephalomyelitis (ME)/chronic fatigue syndrome (CFS)". Physiotherapy. 101: e1091–e1092. doi:10.1016/j.physio.2015.03.1984. ISSN 0031-9406.
- ↑ Cordero, D. L.; Sisto, S.A.; Tapp, W.N.; LaManca, J.J.; Pareja, J.G.; Natelson, B.H. (December 1996). "Decreased vagal power during treadmill walking in patients with chronic fatigue syndrome". Clinical Autonomic Research: Official Journal of the Clinical Autonomic Research Society. 6 (6): 329–333. ISSN 0959-9851. PMID 8985621.
- ↑ Soetekouw, P. M.; Lenders, J.W.; Bleijenberg, G.; Thien, T.; van der Meer, J.W. (December 1999). "Autonomic function in patients with chronic fatigue syndrome". Clinical Autonomic Research: Official Journal of the Clinical Autonomic Research Society. 9 (6): 334–340. ISSN 0959-9851. PMID 10638807.
- ↑ LaManca, J.J.; Peckerman, A.; Sisto, S.A.; DeLuca, J.; Cook, S.; Natelson, B.H. (September 2001). "Cardiovascular responses of women with chronic fatigue syndrome to stressful cognitive testing before and after strenuous exercise". Psychosomatic Medicine. 63 (5): 756–764. ISSN 0033-3174. PMID 11573024.
- ↑ Egge, Caroline; Wyller, Vegard Bruun (December 14, 2010). "No differences in cardiovascular autonomic responses to mental stress in chronic fatigue syndrome adolescents as compared to healthy controls". BioPsychoSocial Medicine. 4: 22. doi:10.1186/1751-0759-4-22. ISSN 1751-0759. PMC 3012010. PMID 21156045.
- ↑ Ocon, Anthony J.; Messer, Zachary R.; Medow, Marvin S.; Stewart, Julian M. (March 2012). "Increasing orthostatic stress impairs neurocognitive functioning in chronic fatigue syndrome with postural tachycardia syndrome". Clinical Science (London, England: 1979). 122 (5): 227–238. doi:10.1042/CS20110241. ISSN 1470-8736. PMC 3368269. PMID 21919887.
- ↑ Togo, Fumiharu; Natelson, Benjamin H.; Cherniack, Neil S.; Klapholz, Marc; Rapoport, David M.; Cook, Dane B. (January 2010). "Sleep is not disrupted by exercise in patients with chronic fatigue syndromes". Medicine and Science in Sports and Exercise. 42 (1): 16–22. doi:10.1249/MSS.0b013e3181b11bc7. ISSN 1530-0315. PMC 2796587. PMID 20010134.
- ↑ Kishi, Akifumi; Togo, Fumiharu; Cook, Dane B; Klapholz, Marc; Yamamoto, Yoshiharu; Rapoport, David M; Natelson, Benjamin H (November 2013). "The effects of exercise on dynamic sleep morphology in healthy controls and patients with chronic fatigue syndrome". Physiological Reports. 1 (6). doi:10.1002/phy2.152. ISSN 2051-817X. PMC 3871467. PMID 24400154.
- ↑ Cvejic, Erin; Sandler, Carolina X.; Keech, Andrew; Barry, Benjamin K.; Lloyd, Andrew R.; Vollmer-Conna, Uté (December 2017). "Autonomic nervous system function, activity patterns, and sleep after physical or cognitive challenge in people with chronic fatigue syndrome". Journal of Psychosomatic Research. 103: 91–94. doi:10.1016/j.jpsychores.2017.10.010. ISSN 1879-1360. PMID 29167053.
- ↑ Ohashi, Kyoko; Yamamoto, Yoshiharu; Natelson, Benjamin H. (September 2002). "Activity rhythm degrades after strenuous exercise in chronic fatigue syndrome". Physiology & Behavior. 77 (1): 39–44. ISSN 0031-9384. PMID 12213500.
- ↑ Marshall, P. S.; Forstot, M.; Callies, A.; Peterson, P.K.; Schenck, C.H. (January 1997). "Cognitive slowing and working memory difficulties in chronic fatigue syndrome". Psychosomatic Medicine. 59 (1): 58–66. ISSN 0033-3174. PMID 9021867.
- ↑ LaManca, J.J.; Sisto, S.A.; DeLuca, J.; Johnson, S.K.; Lange, G.; Pareja, J.; Cook, S.; Natelson, B.H. (September 28, 1998). "Influence of exhaustive treadmill exercise on cognitive functioning in chronic fatigue syndrome". The American Journal of Medicine. 105 (3A): 59S–65S. ISSN 0002-9343. PMID 9790484.
- ↑ Claypoole, Keith; Mahurin, Roderick; Fischer, Mary E.; Goldberg, Jack; Schmaling, Karen B.; Schoene, Robert B.; Ashton, Suzanne; Buchwald, Dedra (March 2001). "Cognitive Compromise Following Exercise in Monozygotic Twins Discordant for Chronic Fatigue Syndrome: Fact or Artifact?". Applied Neuropsychology. 8 (1): 31–40. doi:10.1207/s15324826an0801_5. ISSN 0908-4282.
- ↑ Cook, Dane B.; Nagelkirk, Paul R.; Peckerman, Arnold; Poluri, Ashok; Mores, John; Natelson, Benjamin H. (September 2005). "Exercise and cognitive performance in chronic fatigue syndrome". Medicine and Science in Sports and Exercise. 37 (9): 1460–1467. ISSN 0195-9131. PMID 16177595.
- ↑ Yunus, Muhammad (July 2, 2015). "Editorial Review (Thematic Issue: An Update on Central Sensitivity Syndromes and the Issues of Nosology and Psychobiology)". Current Rheumatology Reviews. 11 (2): 70–85. doi:10.2174/157339711102150702112236. ISSN 1573-3971.
- ↑ Jump up to: 120.0 120.1 Shukla, Sanjay K.; Cook, Dane; Meyer, Jacob; Vernon, Suzanne D.; Le, Thao; Clevidence, Derek; Robertson, Charles E.; Schrodi, Steven J.; Yale, Steven (December 18, 2015). "Changes in Gut and Plasma Microbiome following Exercise Challenge in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS)". PLOS ONE. 10 (12): e0145453. doi:10.1371/journal.pone.0145453. ISSN 1932-6203.
- ↑ Strahler, Jana; Fischer, Susanne; Nater, Urs M.; Ehlert, Ulrike; Gaab, Jens (September 2013). "Norepinephrine and epinephrine responses to physiological and pharmacological stimulation in chronic fatigue syndrome". Biological Psychology. 94 (1): 160–166. doi:10.1016/j.biopsycho.2013.06.002. ISSN 1873-6246. PMID 23770415.
- ↑ Suárez, Andrea; Guillamó, Elisabet; Roig, Teresa; Blázquez, Alicia; Alegre, José; Bermúdez, Jordi; Ventura, José Luis; García-Quintana, Ana María; Comella, Agustí (June 2010). "Nitric Oxide Metabolite Production During Exercise in Chronic Fatigue Syndrome: A Case-Control Study". Journal of Women's Health. 19 (6): 1073–1077. doi:10.1089/jwh.2008.1255. ISSN 1540-9996.
- ↑ Jason, Leonard; Evans, Meredyth (April 27, 2012). "To PEM or not to PEM? That is the question for case definition | Research 1st website". Research first blog. Retrieved October 10, 2018.
- ↑ Jump up to: 124.0 124.1 124.2 Fukuda, K.; Straus, S.E.; Hickie, I.; Sharpe, M.C.; Dobbins, J.G.; Komaroff, A. (December 15, 1994). "The chronic fatigue syndrome: a comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group" (PDF). Annals of Internal Medicine. American College of Physicians. 121 (12): 953–959. ISSN 0003-4819. PMID 7978722.
- ↑ Jason, Leonard A.; King, Caroline P.; Richman, Judith A.; Taylor, Renee R.; Torres, Susan R.; Song, Sharon (January 1999). "U.S. Case Definition of Chronic Fatigue Syndrome". Journal of Chronic Fatigue Syndrome. 5 (3–4): 3–33. doi:10.1300/j092v05n03_02. ISSN 1057-3321.
- ↑ Keech, Andrew; Sandler, Carolina X.; Vollmer-Conna, Ute; Cvejic, Erin; Lloyd, Andrew R.; Barry, Benjamin K. (December 2015). "Capturing the post-exertional exacerbation of fatigue following physical and cognitive challenge in patients with chronic fatigue syndrome". Journal of Psychosomatic Research. 79 (6): 537–549. doi:10.1016/j.jpsychores.2015.08.008. ISSN 1879-1360. PMID 26359713.
- ↑ Jason, Leonard A.; Evans, Meredyth Anne; Porter, Nicole; Brown, Molly; Brown, Abigail A.; Hunnell, Jessica; Anderson, Valerie C.; Lerch, Athena; Meirleir, Kenny de (2010). "The Development of a Revised Canadian Myalgic Encephalomyelitis Chronic Fatigue Syndrome Case Definition". Am J Biochem and Biotech. 6 (2): 120–135.
- ↑ Strand, Elin B.; Lillestøl, Kristine; Jason, Leonard A.; Tveito, Kari; Diep, Lien My; Valla, Simen Strand; Sunnquist, Madison; Helland, Ingrid B.; Herder, Ingrid (January 2, 2016). "Comparing the DePaul Symptom Questionnaire with physician assessments: a preliminary study". Fatigue: Biomedicine, Health & Behavior. 4 (1): 52–62. doi:10.1080/21641846.2015.1126026. ISSN 2164-1846.
- ↑ Murdock, Kyle W.; Wang, Xin Shelley; Shi, Qiuling; Cleeland, Charles S.; Fagundes, Christopher P.; Vernon, Suzanne D. (April 2017). "The utility of patient-reported outcome measures among patients with myalgic encephalomyelitis/chronic fatigue syndrome". Quality of Life Research: An International Journal of Quality of Life Aspects of Treatment, Care and Rehabilitation. 26 (4): 913–921. doi:10.1007/s11136-016-1406-3. ISSN 1573-2649. PMC 5336422. PMID 27600520.
- ↑ Jason, Leonard; Jessen, Tricia; Porter, Nicole; Boulton, Aaron; Gloria-Njoku, Mary (July 16, 2009). "Examining Types of Fatigue Among Individuals with ME/CFS". Disability Studies Quarterly. 29 (3). doi:10.18061/dsq.v29i3.938. ISSN 2159-8371.
- ↑ Jump up to: 131.0 131.1 131.2 131.3 Jason, L. A.; McManimen, S.L.; Sunnquist, M.; Holtzman, C.S. (March 21, 2018). "Patient perceptions of post exertional malaise". Fatigue: Biomedicine, Health & Behavior. 6 (2): 92–105. doi:10.1080/21641846.2018.1453265. ISSN 2164-1846.
- ↑ NINDS CDE Project Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) Post Exertional Malaise Subgroup. "Guidance for Core PEM Assessment" (PDF). Retrieved February 21, 2021.
- ↑ Larun, Lillebeth; Malterud, Kirsti (May 2011). "Finding the right balance of physical activity: a focus group study about experiences among patients with chronic fatigue syndrome". Patient Education and Counseling. 83 (2): 222–226. doi:10.1016/j.pec.2010.05.027. ISSN 1873-5134. PMID 20580520.
- ↑ Behan, P. O.; Behan, W.M.; Bell, E.J. (May 1985). "The postviral fatigue syndrome - an analysis of the findings in 50 cases". The Journal of Infection. 10 (3): 211–222. ISSN 0163-4453. PMID 2993423.
- ↑ Holmes, G. P.; Kaplan, J.E.; Gantz, N.M.; Komaroff, A.L.; Schonberger, L.B.; Straus, S.E.; Jones, J.F.; Dubois, R.E.; Cunningham-Rundles, C. (March 1988). "Chronic fatigue syndrome: a working case definition". Annals of Internal Medicine. 108 (3): 387–389. ISSN 0003-4819. PMID 2829679.
- ↑ Komaroff, Anthony L.; Buchwald, Dedra (January 1, 1991). "Symptoms and Signs of Chronic Fatigue Syndrome". Clinical Infectious Diseases. 13 (Supplement_1): S8–S11. doi:10.1093/clinids/13.Supplement_1.S8. ISSN 1537-6591.
- ↑ Haney, Elizabeth; Smith, M.E. Beth; McDonagh, Marian; Pappas, Miranda; Daeges, Monica; Wasson, Ngoc; Nelson, Heidi D. (June 16, 2015). "Diagnostic Methods for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Systematic Review for a National Institutes of Health Pathways to Prevention Workshop". Annals of Internal Medicine. 162 (12): 834. doi:10.7326/m15-0443. ISSN 0003-4819.
- ↑ Swift, Penny (January 16, 2015). "US NIH Report Calls for UK Definition of ME/CFS to be Scrapped". The Argus Report. Retrieved February 28, 2019.
- ↑ Carruthers, Bruce; van de Sande, Marjorie. "Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Clinical Case Definition and Guidelines for Medical Practitioners - An Overview fo the Canadian Consensus Document" (PDF). Invest in ME Research. p. 4.
Physical or mental exertion often causes debilitating malaise and/or fatigue, generalized pain, deterioration of cognitive functions, and worsening of other symptoms that may occur immediately after activity or be delayed. Patients experience rapid muscle fatigue and lack endurance.
- ↑ Soriano, Joan B.; Allan, Maya; Alsokhn, Carine; Alwan, Nisreen A.; Askie, Lisa; Davis, Hannah E.; Diaz, Janet V.; Dua, Tarun; de Groote, Wouter; Jakob, Robert; Lado, Marta; Marshall, John; Murthy, Srin; Preller, Jacobus; Relan, Pryanka; Schiess, Nicoline; Seahwag, Archana (October 6, 2021), A clinical case definition of post COVID-19 condition by a Delphi consensus, World Health Organization (WHO) clinical case definition working group on post COVID-19 condition, World Health Organization
- ↑ "Symptoms Inventory Questionnaire | Myalgic Encephalomyelitis/Chronic Fatigue Syndrome" (PDF). Centers for Disease Control and Prevention. May 18, 2018. Retrieved November 21, 2018.
- ↑ Lawrie, S. M.; Machale, S.M.; Power, M.J.; Goodwin, G.M. (September 1997). "Is the chronic fatigue syndrome best understood as a primary disturbance of the sense of effort?". Psychological Medicine. 27 (5): 995–999. ISSN 1469-8978.
- ↑ Rosen, S D; King, J C; Wilkinson, J B; Nixon, P G (December 1990). "Is chronic fatigue syndrome synonymous with effort syndrome?". Journal of the Royal Society of Medicine. 83 (12): 761–764. ISSN 0141-0768. PMC 1292947. PMID 2125315.
- ↑ Wallman, Karen E.; Sacco, Paul (January 2007). "Sense of effort during a fatiguing exercise protocol in chronic fatigue syndrome". Research in Sports Medicine. 15 (1): 47–59. doi:10.1080/15438620601184331. ISSN 1543-8627. PMID 17365951.
- ↑ Silver, A.; Haeney, M.; Vijayadurai, P.; Wilks, D.; Pattrick, M.; Main, C.J. (June 2002). "The role of fear of physical movement and activity in chronic fatigue syndrome". Journal of Psychosomatic Research. 52 (6): 485–493. ISSN 0022-3999. PMID 12069873.
- ↑ Fischler, B.; Dendale, P.; Michiels, V.; Cluydts, R.; Kaufman, L.; De Meirleir, K. (April 1997). "Physical fatigability and exercise capacity in chronic fatigue syndrome: association with disability, somatization and psychopathology". Journal of Psychosomatic Research. 42 (4): 369–378. ISSN 0022-3999. PMID 9160276.
- ↑ Nijs, J; De Meirleir, K; Duquet, W (2004). "Tampa Scale Kinesiophobia - Version Chronic Fatigue Syndrome" (PDF). painmotion.be. Archives of Physical Medicine and Rehabilitation.
- ↑ "Definition of malaise in English by Oxford Dictionaries". Oxford Dictionaries | English. Retrieved October 13, 2018.
- ↑ Tuller, David (November 23, 2011). "Chronic Fatigue Syndrome and the CDC: A Long, Tangled Tale". Virology blog. Retrieved October 10, 2018.
- ↑ Bond, Joshua; Nielsen, Tessa; Hodges, Lynette (2021). "Effects of Post-Exertional Malaise on Markers of Arterial Stiffness in Individuals with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome". International Journal of Environmental Research and Public Health. 18 (5): 2366. doi:10.3390/ijerph18052366. ISSN 1660-4601. PMC 7957494. PMID 33671082.
- ↑ Mateo, Lariel J. (2018). "Comparing Post-Exertional Symptoms Following Serial Exercise Tests". PURCC – via Scholarly Commons.
- ↑ Lindheimer, Jacob B.; Meyer, Jacob D.; Stegner, Aaron J.; Dougherty, Ryan J.; Van Riper, Stephanie M.; Shields, Morgan; Reisner, Amanda; Shukla, Sanjay K.; Light, Alan R. (April 3, 2017). "Symptom variability following acute exercise in myalgic encephalomyelitis/chronic fatigue syndrome: a perspective on measuring post-exertion malaise". Fatigue: Biomedicine, Health & Behavior. 5 (2): 69–88. doi:10.1080/21641846.2017.1321166. ISSN 2164-1846.
- ↑ Jason, LA; Zinn, ML; Zinn (September 2015). "Myalgic Encephalomyelitis: Symptoms and Biomarkers". Current Neuropharmacology. 13 (5): 701-734. doi:10.2174/1570159X13666150928105725.
- ↑ Jason, Leonard A.; Sunnquist, Madison; Brown, Abigail; Furst, Jacob; Cid, Marjoe; Farietta, Jillianna; Kot, Bobby; Bloomer, Craig; Nicholson, Laura (September 2015). "Factor Analysis of the DePaul Symptom Questionnaire: Identifying Core Domains". Journal of neurology and neurobiology. 1 (4). ISSN 2379-7150. PMC 4830389. PMID 27088131.
- ↑ Paul, L.; Wood, L.; Behan, W.M.; Maclaren, W.M. (1999). "Demonstration of delayed recovery from fatiguing exercise in chronic fatigue syndrome". European Journal of Neurology. 6 (1): 63–69. ISSN 1351-5101. PMID 10209352.

